
Did Spiders Evolve Knees Through Gene Duplication?
News to Know
Abstract
Should creationists fear the walking spider, knee-deep in evolution?
News Sources
- Science: “How Spiders Got Their Knees”
- Christian Science Monitor: “How the Spider Got His Knees”
- EurekAlert!: “Knee-Deep in Spider Leg Evolution”
Spiders and other arachnids have knees—though no kneecaps. Their knees, like ours, connect longer leg parts, but spider knees can move much more freely.
What good are knees to a spider? After all, other arthropods—insects, lobsters, centipedes, and so forth—get along without them. All have segmented legs, but only arachnids—the subgroup that includes spiders, scorpions, and mites—have knees. The arachnid knee, or patella, is short and bell-shaped. Interspersed between longer segments, the knee increases overall limb flexibility. The arachnid knee makes it possible for the leg below it to move independently in almost any direction.
Dachshunds and Spider Knees
While trying to learn why some spiders have longer legs than others, scientists from Germany’s University of Göttingen were surprised to discover that spiders have not one but two dachshund genes. The dachshund gene was named after the stumpy-legged wiener dog bred to poke its long body into badger holes. The dachshund (dac) gene was originally discovered in fruit flies, which have only one, and dac-mutant flies have short, malformed legs.1 The spider’s dachshund genes don’t affect the length of its legs, but one of them, it turns out, gives the spider its knees!
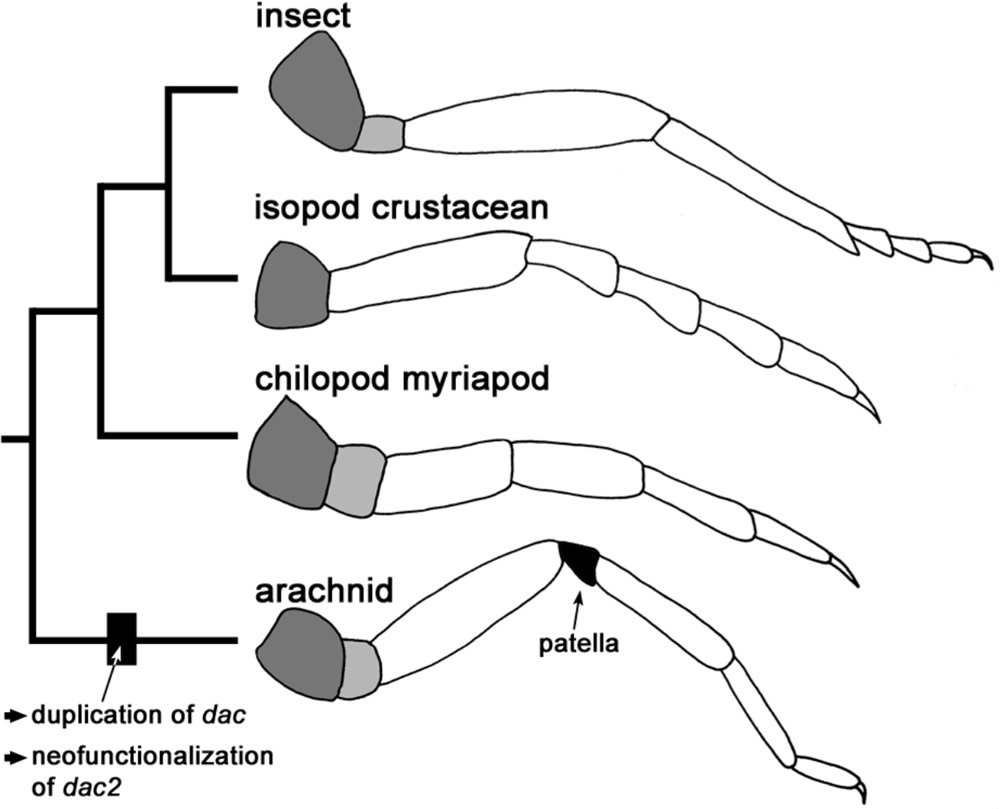
As shown in this diagram, insects, crustaceans, and centipedes—whose legs are shown top to bottom—do not have knees. Arachnids—like spiders, scorpions, and mites"—can move their legs more freely because they have a short bell-shaped patella (knee) connecting two longer leg segments. Image reproduced from N. Turetzek et al., “Neofunctionalization of a Duplicate Dachshund Gene Underlies the Evolution of a Novel Leg Segment in Arachnids,” Molecular Biology and Evolution (October 6, 2015), doi:10.1093/molbev/msv200.
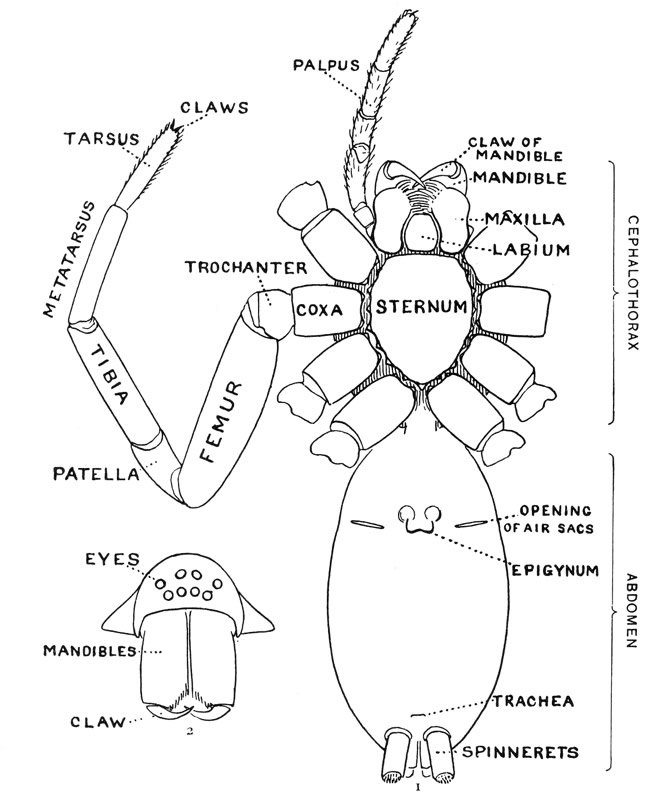
A spider’s leg has 7 segments, labelled here on this sketch of a spider’s underside. Though arthropods have exoskeletons instead of bones, some segments are assigned the same names as vertebrate bones. The patella is the spider’s knee. Connecting two much longer segments (the femur and tibia), the patella makes the spider’s legs more maneuverable than an insect’s, which are knee-less. Reprinted from The Common Spiders of the United States (1902).
The two spider versions of the dachshund gene are called dac1 and dac2. The dac1 gene is expressed in multiple leg segments throughout the spider embryo’s development. The dac2 gene, however, is most keenly expressed in the embryo’s knees, and it is there that its loss is dramatically manifested.2 The scientists discovered that dac2 is essential to knee formation and to the spider’s survival.

Image courtesy of Dr. Elizabeth Mitchell.
Ironically, although it was named for the short-legged dachshund historically bred to hunt burrowing animals, neither the dachshund (dac) gene originally found in fruit flies nor the vertebrate dachshund (dach) gene3 is responsible for this dog’s short legs. Dachshunds do have short legs, however, because of a real copying mutation. This mutation duplicated the gene FGF4 (fibroblast growth factor) and reinserted it at a different place in the dog genome. This gene duplication causes bone growth plates to harden prematurely. The resulting dwarfism, a desirable trait enabling mutant dogs to pursue a badger into its burrow, was maintained in the dachshund by selective breeding. This copying mutation did not add information to the genome but, by preventing normal growth, produced a characteristic phenotypic effect. Of course, this mutation should in no way be considered an example of upward evolution. Not only did the duplication mutation fail to increase the genetic information in the dog genome, but it was maintained in the genome through selective breeding rather than random chance processes, and the dogs—though they are mutant mutts—are and will remain dogs.


These are the two species of spiders whose knees and genes were studied by the German team. Parasteatoda tepidariorum is one of the 32,500 species of entelegyne spiders. Pholcus phalangioides is one of the 3,500 species of haplogyne spiders. These are the two largest groups of spiders.4 Image reproduced from N. Turetzek et al., “Neofunctionalization of a Duplicate . . . ” doi:10.1093/molbev/msv200.
When scientists deactivate the dac2 gene, the knee develops not as a short, freely mobile, bell-shaped segment, but rather as a malformed bump fused to the segment below it. The resulting stiff-legged spider nymphs cannot walk properly. They are readily cannibalized by their normal siblings.5 The survivors die during the second stage of their molting process, unable to extricate their fused knobby-kneed legs from their outgrown exoskeletons.
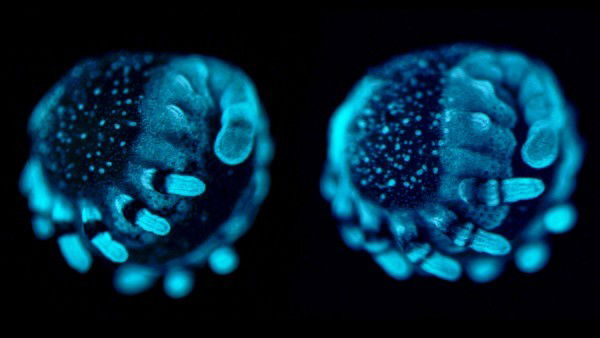
Spider knee development depends on one of its dachshund genes. A dachshund gene makes a transcription factor that helps regulate expression of other genes during embryonic development. The spider embryo’s legs form properly as the spider’s two versions of the dachshund gene are expressed at the proper location and time during embryonic development. The black bands on the legs of the spider embryo on the left indicate dac1 expression, which is essential for forming many of its leg segments. The narrow black band on each leg of the spider embryo on the right indicates the site of dac2’s most intense expression—the developing knees. Spider knees only develop properly if dac2 is expressed during embryonic development at this location. Image credit Nikola-Michael Prpic Göttinger Zentrum für Biowissenschaften, Universität Göttingen, Germany, via Science.
Evolutionary Throwbacks?
The evolutionary researchers consider dac2 a duplicate of dac1. They believe an ancient ancestor took a major evolutionary step toward becoming a spider by putting an accidentally duplicated copy of its dachshund gene to work growing knees.
The researchers are convinced that their deformed spiders represent the anatomy of a knee-less pre-spider. They write, “Removing dac2 function experimentally restores the segment composition of the arthropod leg before the origin of dac2 in the arachnid lineage.”6 Leaping to the conclusion that a duplicated dachshund gene must have acquired a brand new function critical to spider evolution, they add, “In this way, we are able to provide a link between the origin and neofunctionalisation of dac2 and the evolution of a morphological novelty in the arachnid appendage.”7
The authors of the study, published in Molecular Biology and Evolution, make a poor choice of words in calling dac2 a “duplicate gene.” Their terminology and their presumption that dac2 arose through random mutation followed by acquisition of a new function result not from scientific observations but from the authors’ evolutionary worldview.

This is a normal fruit fly. Its legs have ten jointed segments. Like other insects, it does not have a short knee segment connecting its longer segments. Fruit flies with a mutation in the dachshund (dac) gene are typically eyeless, and the middle segments of their unusually short legs are misshapen and fused. Though they flail their abnormal legs, they are unable to walk properly and soon die. Image credit André Karwath via Wikimedia Commons.
Dachshund genes are identified on the basis of sequence similarities to genes in mice and fruit flies.8 Spider dachshund genes are much more like the fruit fly’s. But the two versions of the dachshund gene in spiders are far from identical. The dac2 gene contains a sequence that codes for a chain of 30 amino acids not found in dac1. Furthermore, the so-called duplicate dac2 ends earlier than dac1. Given these dissimilarities and the fact that spider knees do not form without dac2 and that their knees are not just enhancements but are necessary for survival, the evidence suggests that dac2 was part of the spider’s original design. It would be better to reserve the word duplicate for cases in which a copying mutation is clearly evident.
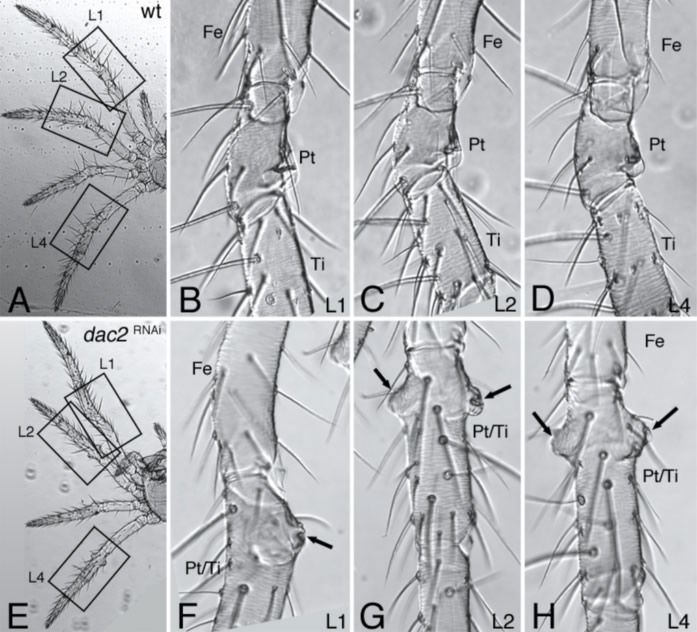
The photographs at the top show the normal spider legs of Parasteatoda tepidariorum, a common house spider. The close-ups in B-D show the normal knee (patella, Pt) connecting the spider’s femur (Fe) and tibia (Ti) on three of the pictured legs (L1, L2, and L4). The photographs on the bottom row show the abnormal legs in a spider whose dac2 gene was inactivated. In place of the distinctive bell-shaped patellas seen in normal legs, this spider’s patellas (Pt/Ti, indicated by arrows) are misshapen bumps. Fused to the tibia, such a patella robs the spider of its usual highly maneuverable lower appendage. Evolutionary research believes this deformed appendage represents an earlier evolutionary form of the spider’s leg. Image reproduced from N. Turetzek et al., “Neofunctionalization of a Duplicate . . . ” doi:10.1093/molbev/msv200.
Dachshunds and Duplicates
Multiple copies of genes—with or without variations—may result from random mutations or be a designed feature of an organism. If functional, they may enhance an existing function. This has been seen in Salmonella bacteria that more efficiently manufacture the amino acid tryptophan if they have duplicate copies of the necessary gene. (See “New Function through Gene Duplication” to learn more.) Duplicates also may affect the expression of existing genes. This is seen in E. coli bacteria that are better able to utilize citrate as a fuel if a duplicate copy of the gene governing citrate transport is located close enough to its regulatory region to keep the gene switched on. (Learn more about this in “De-Regulation of an Existing Trait.”)
Duplicate genes may alter the properties of proteins in a way that ultimately serves other purposes for the organism. This seems to be the case with snake venom. Toxic components of some snake venoms are associated with duplications of the genes that produce nontoxic substances used elsewhere in the body. (You can read more about this in “Evolution of Snake Venom: A New Use for Old Genes?” and “Mamba Venom Derives from Gene Duplication.”)
Evolutionists believe gene duplication is a mechanism by which increasingly complex organisms evolved.
Evolutionists believe gene duplication is a mechanism by which increasingly complex organisms evolved. They believe that multiple versions or copies of a gene—even if they vary—must be the result of copying mutations. And they believe that the use of those genes for different purposes is evidence of neofunctionalization in the gene’s primordial past. Neofunctionalization is the process by which a duplicated gene supposedly acquired a new function in the evolutionary past. “Species constantly adapt and evolve by inventing new body features,” says team leader Nikola-Michael Prpic. “Our work shows how a gene can be duplicated and then used during evolution to invent a new morphological feature.”9
But have the researchers shown that dac2 invented a new anatomical feature in a knee-less ancestor? No. They’ve only shown that spiders have two dachshund genes, that one of them (dac2) is necessary for embryonic knee formation, and that spiders don’t survive without knees.
Neither duplicate genes nor similar genes are proof that they and their functions came to exist through random evolutionary processes. Duplicate copies of a gene, when they do occur, contain the same information, just as a hundred copies of a book contain no more information than one. Even if the hundred books are used in different ways—some to prop open doors, some to decorate coffee tables, some to smack intrusive spiders, and some to educate children—the information in the hundred books is the same as the information contained in just one.
Furthermore, the scientists have not discovered how the information for producing spider knees came to exist in the first place. Neither have they observed neofunctionalization. As we’ve already pointed out, dac2, though similar to dac1, has substantial structural differences. In fact, even their claim that dac2 has a function completely different from dac1 is quite a stretch!
Here’s why. The dachshund genes in fruit flies and in vertebrates make transcription factors that help regulate expression of other genes during embryonic development.10 Thus dachshund genes have essentially the same regulatory function—a regulatory one—but produce different results depending on where and when they are expressed in the embryo. Rather than claiming dac2 evolved a new function—something that has never been observed—it would be more accurate to say that in spiders this regulatory gene is strongly expressed in the knee and controls correct development there, just as dac1 controls correct development of the leg overall.
Embryologic Development Versus Evolution
The deformity produced by the deactivation of dac2 is not a picture of the spider’s evolutionary past. It is only observational proof of the gene’s essential role in the present. Scientists can learn about how the gene governs spider knee development in the present. They cannot observe how spiders hypothetically evolved in the past.
Observable embryologic development is not a reliable roadmap to an unobservable evolutionary past.
Evolutionists look for clues to explain the origin of signature traits like spider knees in their embryologic development. Embryonic development, because it lays out the steps that actually work in real time to form a single animal, helps evolutionists come up with a plausible sequence of changes that would be required if evolution of one kind of animal into a more complex one could occur. However, observable embryologic development is not a reliable roadmap to an unobservable evolutionary past.
Two functional versions of a gene do not demonstrate that the organism possessing them evolved through gene duplication with neofunctionalization. The alternative explanation is that two similar genes, dac1 and dac2, were designed to ensure formation of functional legs with knees in spiders. Of course this is not acceptable to evolutionists who presuppose that random naturalistic processes of molecules-to-man evolution are the source of all life.
Furthermore, the genetic underpinning of knees in other arachnids varies. Mites have knees but only one known dachshund gene, whereas scorpions have knees and have three dachshund genes, all much shorter than those in spiders. Each was designed with knees and the genes to form them. To try to connect the evolutionary dots between different kinds of animals through genetic similarities is a purely imaginary pursuit.
God Designed the Spider’s Knees
We know how the spider got its knees. God designed spiders with knees. It is delightful to see scientists unravel the genetic mechanism He designed in order to equip all spiders with them. God even commented on the lowly spider’s resulting dexterity in His Word:
The spider skillfully grasps with its hands,
And it is in kings’ palaces. (Proverbs 30:28)
So the next time you see a spider nimbly scuttling about in your palace or garden, perhaps meticulously manipulating its web or the things caught in it, consider how its tiny bell-shaped knees, a gift from its Creator, our common designer and a master engineer, have for 6,000 years equipped spiders for their unique lifestyle.
Further Reading
- Fear the Parasitic Wasp: Spider Zombies Caught in Web of Deceit
- Bolas Spiders—Eight-Legged Gauchos
- Scorpion Venom Is No Match for Mighty Mouse
- New Function through Gene Duplication
- Creepy Crawlies
For More Information: Get Answers
Remember, if you see a news story that might merit some attention, let us know about it! (Note: if the story originates from the Associated Press, FOX News, MSNBC, the New York Times, or another major national media outlet, we will most likely have already heard about it.) And thanks to all of our readers who have submitted great news tips to us. If you didn’t catch all the latest News to Know, why not take a look to see what you’ve missed?
(Please note that links will take you directly to the source. Answers in Genesis is not responsible for content on the websites to which we refer. For more information, please see our Privacy Policy.)
Footnotes
- The dachshund (dac) gene was named for the short-legged dog because the intermediate leg segments of dac-mutant fruit flies are fused and misshapen, and the mutant fruit flies’ legs are quite short. However, the dachshund (dac) gene is involved in regulating the expression of other genes too. Therefore, dac mutations also have other effects, including eyelessness in fruit flies.
- The dac1 gene is strongly expressed in multiple leg segments throughout the spider embryo’s development as well as in its pedipalps (specialized sensory organs formed from the second set of legs), central nervous system, and heart. The pattern of dac2 gene expression in the embryonic spider differs. While dac2 is expressed in the legs, pedipalps, and even the lateral portion of the eyes, the gene’s expression is greatly intensified in the knees. Without dac2, spider knees cannot develop properly, and the experimentally damaged spiders do not survive. From N. Turetzek et al., “Neofunctionalization of a Duplicate . . .” doi:10.1093/molbev/msv200.
- The dachshund gene in vertebrates (dach) makes a protein that works with other transcription factors to regulate the expression of other genes for producing legs and internal organs. In mice, dach is expressed during limb and eye development. Impairment in one of the two human dachshund genes is associated with some aggressive metastatic cancers.
- Two smaller groups of spiders, the mygalomorphs (2,500 species including tarantulas) and mesothelae (containing about 100 living species), were not included in this study.
- Since dac2 is also expressed in the lateral part of the embryonic eyes, we might wonder whether the mutant spider nymphs actually saw their destruction coming.
- N. Turetzek et al., “Neofunctionalization of a Duplicate . . .” doi:10.1093/molbev/msv200.
- Ibid.
- Homologous genes—similar genes present in a variety of species—are an example of the common designs we would expect from a wise common Designer, our Creator, who created all things great and small. Many genes have homologous corresponding versions present in the genomes of many animals and people. The existence of similar and even matching genes does not explain their origin, however. Genetic similarities represent one of the many ways in which we see that God used similar designs—whether molecular or anatomic—in various ways throughout the biological world.
- “Knee-Deep in Spider Leg Evolution,” EurekAlert, October 6, 2015, http://www.eurekalert.org/pub_releases/2015-10/mbae-kis100115.php.
- As with many genes, the function of the dachshund family of genes is still under investigation, so it is not surprising that the specific functions in spiders are just now being worked out. In another invertebrate, the fruit fly, however, the dachshund gene (dac), through the transcription factor it encodes, works with other genes to control expression of other genes including those that form the eye, limbs, and genitalia. Humans have two dachshund genes, dach1 and dach2. They also make transcription factors that work with other transcription factors to regulate cellular proliferation during development. Dach1 is located on chromosome 13, and its failure later in life is associated with some forms of aggressive cancer. Dach2 is located on the X-chromosome, regulates embryonic development of organs and muscles, and is related to premature ovarian failure. From C. Brás-Pereira et al., “The Retinal Determination Gene Dachshund Restricts Cell Proliferation By Limiting the Activity of the Homothorax-Yorkie Complex,” Development 142 (2015): 1470–1479, doi:10.1242/dev.113340, and the National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/gene/1602 and http://www.ncbi.nlm.nih.gov/gene/117154.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


