Attempts to Trace Life Back to Chemical Origins Still Maps the Willful Ignorance of the Hunters
News to Know
Abstract
Trickle-down chemistry supposedly solves the chemical conundrum concerning the origin of life, but molecules-to-man evolution remains as fictional and imaginative as ever.
News Sources
- Science: “Researchers May Have Solved Origin-of-Life Conundrum”
- ScienceNews: “Cooking Up Life’s Ingredients, All in One Pot”
- Mapping Ignorance: “Living Beings: Systems All the Way Back to Their Chemical Origins”
Editor’s note: This article presents the latest claims concerning the evolutionary origin of life and, updating a previous article on the subject, points out that the supposed answer is no answer at all.
Riddle-solving researchers from the University of Cambridge “may have solved origin-of-life conundrum,” announced a recent headline. They propose a scenario by which life’s essential chemical building blocks could have been produced simultaneously, providing the raw material for life to evolve. John Sutherland’s Cambridge team resolved this foundational chemical conundrum using simple molecules they contend would have been deposited on the early Earth by heavy meteorite bombardment.1
“The key thing about the network is that although it looks complicated, it’s all the same reactions,” Sutherland explains.2 From hydrogen cyanide, hydrogen sulfide, phosphates, and of course water, the organic building blocks of many important biological molecules can form. The same sorts of reactions, using various metallic catalysts, can produce 12 amino acids, 2 nucleotides, and a lipid precursor. Thus, using the sun’s energy, it is possible to generate many of the simple molecules from which the far more complex biochemical molecules comprising living cells are built. That would “only” leave the problem of getting those molecules to assemble and organize themselves into living cells, but more on that later.
While the fact that all these reactions involved moving electrons around on carbon-containing molecules makes the reactions a common sort of chemistry, it does not make them simple in the sense of “ready to happen all on their own.” Lest this scenario and Sutherland’s suggestion that these reactions are uncomplicated because they are “all the same” leave readers with the impression that the researchers just sat back and watched these reactions unfold, we must point out that the researchers had to repeatedly intervene by sprinkling in additional reactants, replenishing the original substrates (starter chemicals), or adding energy in the form of heat or irradiation.
The researchers claim that these are things that would have randomly happened on their idea of an early Earth in a myriad of trickling streams of water flowing down countless hillsides exposed to the sun. The only difference in the story they weave and that of other scenarios proposed beginning with the Miller-Urey experiment is that they claim to have a way to “allow” these reactions to take place in separate trickling streams so that they won’t interfere with each other, later combining the products at the bottom of a hill.
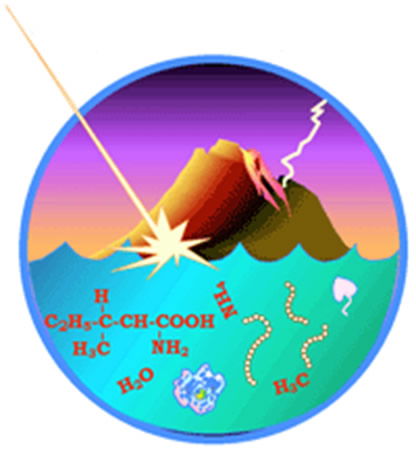
How could simple molecules evolve through natural processes into living cells? They could not. Neither on Earth after trickle-down chemistry spawned the necessary building blocks as Cambridge researchers assert3 nor on an extraterrestrial world such as Saturn’s moon Titan, as Cornell University scientists have recently claimed.4 (Exporting the biological and informational deficiencies of evolution to space does nothing to resolve them.) Natural processes cannot explain the origin of life. Evolutionists grasp for explanations in vain, willfully ignoring the eyewitness origins of history provided by the Author of life. Image reproduced from NASA through Creation Wiki.org.
One-Pot Problem “Solved”
Evolutionary efforts to envision abiogenesis have been hampered by the need to cook up all life’s building blocks in one pot. Recalling the failings of the famous Miller-Urey experiment and other one-pot approaches, Sutherland’s team decided it “would be difficult”5 for any of life’s essential chemicals to survive exposure to the conditions needed to produce the others. The reactions interfere with each other if they all happen in the same pot.
Sutherland’s group therefore came up with a different sort of story to carry out the chemistry to get from simple inorganics provided by meteorites to Darwin’s “warm little pond.”6 Though published in Nature Chemistry with considerable details of the chemistry—and intervention—required to making each step work out just so, each journalist describing the Sutherland’s vision of how life came from random chemical reactions naturally paraphrased that story for public consumption. Rather than quoting the way others have boiled the story down to layman’s terms, I prefer to paraphrase it in layman’s terms this way. The latest story offered to solve the abiogenesis conundrum, then, goes something like this:
A long time ago on a world very different from the one we see today, meteorite bombardment left some simple chemical compounds on hilltops. Later as the rain came down little rivulets began flowing downhill, dissolving and carrying away some of those chemicals and picking up others along the way. And in each of those rivulets a spontaneous chemical reaction started. Not all the chemical reactions were the same, but in each rivulet the reaction was able to keep going because the flowing water carried away the reaction products so the original reactants could make more. Some rivulets picked up additional chemical compounds from the terrain over which they flowed, providing raw materials for another chemical reaction.
Finally the rivulets, each carrying a precious organic compound, flowed into the same ponds and puddles of brine in basins at the bottom of the hills. There the different organic compounds that had formed during the journey of the small streams that trekked across terra firma began to bump together and interact, and every now and then a reaction happened that produced a more complex compound that would be an important component of the living cell.
The next stage in the story—coming attractions in the wake of this elegant solution—could be summed up like this:
One day the right chemicals bumped together and somehow the original building blocks from the rivulets were able to interact through random processes in such a way that a lipid membrane wrapped around some chains of nucleotides and amino acids. Now packaged together with inheritable genetic material, the first primordial cell was ready to begin its evolutionary journey. It made copies of itself, and life had gotten the go-ahead to evolve. And all because water flowed downhill in separate streams that simultaneously facilitated the spontaneous chemical reactions that produced the most basic building blocks of life.
Impossible Plausibility
Like most fictional stories, this one is best enjoyed if it is plausible enough to allow us to suspend our disbelief. If we believed that the Earth actually existed more than four billion years ago and was bombarded in the wake of the explosive natural processes that birthed the planets, then some of the chemistry might be plausible. (Of course, it is easy enough to imagine what the early Earth might have been like at any given stage if you disregard the only historical eyewitness record we have of that time—God’s eyewitness account in Genesis, where we learn that Earth was made ready to support the life God created for it in just a few 24-hour days. Nothing in the historical record supports the sort of imaginary Earth evolutionists envision.) Energy in the form of ultraviolet radiation from the sun is believable enough, as is the eventual existence of rain in this scenario, though evolutionists remain divided about the origin of Earth’s water. And the idea that water streaming downhill could imitate industrial chemistry—segregating the reactions, keeping production going by siphoning away the product, collecting additional reactants or catalysts from the ground beneath, and finally dumping the whole set of products into the primordial stew—makes the whole thing sound like a dandy tale concocted specifically to preclude the obvious.
But does arguably plausible chemistry on a hillside long ago explain how life could spring into being on its own and how the living cell created itself through random processes? How the vast amount of information encoded into the genome of even the simplest cell came to exist without either a Designer of information or a Creator of the chemical code that carries it from generation to generation? Or how irreducible complexity was conquered as each part of the interrelated systems in the cell supposedly came to be without the presence of all the other parts on which they depend? No. It does none of that. It is just a little story. It does not answer the riddle of life’s origins.
To believe that life could generate itself spontaneously from nonliving molecules through random natural processes flies in the face of observational science, for nothing in experimental biology has ever shown that abiogenesis—life from non-life—is possible.
Goo-to-you, molecules-to-man, pond-scum-to-puddy-tats7—these terms refer to abiogenesis, the essential starting point for evolution of life through natural processes. Yet in a massive review published in 2014 in the American Chemical Society’s Chemical Reviews, researchers report, “The origin of life is a fascinating, unresolved problem.”8 Transplanting the problem to hydrothermal vents in the “Lost City” to take advantage of the catalytic properties of surface minerals didn’t solve the problem. Neither has the latest story from Cambridge. Recent claims from Cornell University, after identifying molecules whose properties could allow them to behave like the phospholipid molecules making up cellular membranes, assert that they have devised “the first concrete blueprint of life not as we know it.”9 But they have only transported the problems with chemicals-to-cells evolution to space, not solved the mystery of life’s origins. And the mystery will remain for all who fail to acknowledge God’s eyewitness account of the origin of life. You see, there is a book . . . Well, before we get to that, let’s take a closer look at that mystery and have a whirlwind tour of its suggested solutions.
Unsolved Mystery
Cosmos host Neil deGrasse Tyson, in the first episode of the new series that premiered in 2014 said, “The origin of life is one of the greatest unsolved mysteries of science.” Scooping up some water, Tyson added, “That’s life cooking, evolving all the biochemical recipes for its incredibly complex activities.”10 Yet while evolutionary scientists, educators, and television personalities promote supposed transitional forms, if they cannot get living cells to evolve from non-living elements through natural processes, their supposed evolutionary extravaganza is over before it starts.
In “Prebiotic Systems Chemistry: New Perspectives for the Origins of Life,” Ruiz-Mirazo and colleagues, biophysicists and biochemists specializing in molecular evolution, spend 82 pages detailing all the things that have failed to demonstrate how life began on its own. They explain that working out plausible pathways to get the chemical components of life to perform in some life-like ways—self-replication, or glomming together in an organized fashion, or wrapping themselves into a membrane-like bubble—isn’t enough. Rather, chemists need to analyze ways in which all the needed chemical accomplishments can happen at once.
This “system integration approach,” according to the authors, is essential if they are to have any hope of showing how the first cell evolved. Simply put, they envision that once upon a time, when countless interdependent chemical processes happened to strike the right combination, the first cell popped into being with all the biochemical basics in place, functioning properly, and able to reproduce itself.
What Hasn’t Worked
Abiogenesis has never been observed in experimental biology and violates the most fundamental law in biology, the law of biogenesis. Nevertheless, the authors of the review are confident there was a naturalistic chemical origin for life. Their tome, however, makes one wonder what side of the argument they’re on. Statement after statement describes why all approaches thus far have been inadequate, emphasizing the staggering complexity of the problem. “Even the simplest microorganisms known on Earth are breathtakingly complex,” they write. “Indeed, the probability that a random sequence of physicochemical events would lead to a bacterium by spontaneous self-organization of biomolecules is negligibly low”11 (emphasis added).
Just showing how membranes could spontaneously assemble and wrap around evolving protocells on a primitive Earth has proven prohibitive. The authors admit, “Despite the pioneering work of Hargreaves et al. in 1977, who demonstrated that the synthesis of phosphatidic acid and other lipids could be achieved abiotically, it is considered very improbable that fatty acids, glycerol, and phosphate (i.e., the standard molecular components of a phospholipid) could have been present together in high enough concentrations on the primordial Earth.”
Membranes aren’t the only missing ingredient. For instance, the authors say, “Successful chemistries for synthesizing nucleobases, sugars, and condensed phosphates, under primordial conditions, have been developed over the last 40 years, though most of the individual synthetic steps face difficulties.”
Listing many other unanswered questions—such as “Where on Earth did life emerge? Or did life arrive here from some extraterrestrial source instead? Did life originate only once, by accident, or is it the probable outcome of chemical evolution, which has frequently occurred elsewhere in the universe? Which property of living beings came first: their ability to reproduce and transmit information to progeny, their metabolic capacity, or their compartmentalization as individual entities?12—the authors admit that scientists cannot even know what the original conditions were on Earth when life supposedly evolved. They write, “Resolving these questions and other related ones is extremely complicated because of our lack of knowledge about the conditions existing on Earth more than 3.5 billion years ago” (emphasis added).
Researchers search for the origin of life in a “pool of simple molecules” like ammonia and hydrogen cyanide struck by sparks, various soups of amino acids, in “RNA world,” “pre-RNA world,” “lipid world,” and “nucleopeptide world,” and through the gift of “extraterrestrial infall.”13 Nevertheless, the authors in Chemical Reviews write, “None of these views has yet gained a unanimous preferential position over the others, each having its own shortcomings. When these various difficulties are considered, it is unlikely that scientists will ever know which exact synthetic itinerary led to the first forms of life.”
It is unlikely, the authors indicate, that any experimental model can truly test the capability of evolutionary models to produce living cells from nonliving elements. They write, “The combination of complex chemical but still infrabiological [non-living] systems . . . into a living entity was probably the main challenge that supramolecular assemblies had to solve during the process of the origins of life. Having said that, integrative experimental approaches are also difficult in the laboratory, since many, not always compatible chemical and physical events need to be coupled in time and space, making diverse types of compounds and dynamic structures come together.”
Summing up the challenges of experimentally creating life in imitation of the supposedly random natural processes by which life created itself, they write, “From a chemical standpoint, the pursued ideal case would be the construction of a living cell from scratch, that is, by self-organization of its most basic molecular building blocks. This is actually what must have happened on the prebiotic Earth, when the first or most primitive cell-like structures appeared and developed into increasingly complex cellular organizations, without any cognitive agent (e.g., an experimental biologist) witnessing the process or interfering with it.”
The Crux of the Problem
Cells are the building blocks of life. And while in their review the authors note that even imagining that viruses qualify as life-forms hasn’t helped unravel the mystery of life’s origin, they see the nature of the cell itself as the reason science has failed thus far in its quest. Ruiz-Mirazo writes,
Cells, as everybody knows, are made of those complex organic molecules; yet the latter come to existence and play a role by virtue of an intricate web of dynamic inter-relationships and transformation processes, which in practice holds all of them together. Remarkably, most biomolecules (except for DNA) have relatively short ‘lifetimes’, much shorter than the lifetime of the collective entity they integrate. Each living organism is a molecular factory in continuous turnover, whose far from equilibrium activity is maintained precisely thanks to the generation of all those structures that are required to build the factory up and keep it running. Without the factory the various pieces, their sheer existence and purpose, just would not make sense: each is produced and has a function in the context of the activity of all the others. If this fact is seriously assimilated, it becomes obvious that the first appearance of something resembling a living being could not be thanks to molecules of a single type. It would be easier to study if it were in that way, no doubt, but life is hard — also for researchers in the field of origins.14
After this ode to the irreducible complexity of the living cell, Ruiz-Mirazo expresses confidence that modern chemistry will ultimately solve the problem. “Scientists interested in the chemistry-biology gap will find, at last, a natural bridge between the two,” he says, enabling us to “cross that river, deep and wide, that still separates us from our chemical roots” knowing that “Nature became a ‘perfect queen’ in these affairs long ago: the integration of diverse molecular components performing different tasks within a complex organization surely started at a proto-cellular stage, long before natural selection pushed the birth of more robust, full-fledged living cells — made of DNA, RNA and proteins.”
Yet these authors still overlook the key component, the element evolutionists are unable to conjure through naturalistic processes: Information. Whether information to become alive in the first place, or information to naturalistically progress all the way up the tree of life, evolutionary science suffers from an appalling lack of information.
What is the source of the information evolutionists seek in vain? Answers in Genesis molecular geneticist Dr. Georgia Purdom has summed it up well:
The river between non-life and life is indeed "deep and wide" and cannot be crossed by a deeper or better understanding of biology and chemistry. Information is required for life and without an information-giver life cannot come about by random chance from a chemical soup. As researchers continue to search for that “magic bullet” to understand the origin of life, they will continue to be frustrated and in their rebellious hearts fail to acknowledge that life can only come from the one true Life-Giver, the Creator God.
Further Reading
For More Information: Get Answers
Remember, if you see a news story that might merit some attention, let us know about it! (Note: if the story originates from the Associated Press, FOX News, MSNBC, the New York Times, or another major national media outlet, we will most likely have already heard about it.) And thanks to all of our readers who have submitted great news tips to us. If you didn’t catch all the latest News to Know, why not take a look to see what you’ve missed?
(Please note that links will take you directly to the source. Answers in Genesis is not responsible for content on the websites to which we refer. For more information, please see our Privacy Policy.)
Footnotes
- They write, “Evidence suggest that life started during, or shortly after the abatement of, the Late Heavy Bombardment, and processes associated with meteorite impact have been implicated in the generation of hydrogen cyanide and phosphate on the Hadean Earth.” From B. Patel et al., “Common Origins of RNA, Protein and Lipid Precursors in a Cyanosulfidic Protometabolism,” Nature Chemistry 7 (March 16, 2015): 301–307, doi:10.1038/nchem.2202.
- Beth Mole, “Cooking Up Life’s Ingredients, All in One Pot,” ScienceNews, March 16, 2015, https://www.sciencenews.org/article/cooking-life’s-ingredients-all-one-pot.
- Robert F. Service, “Researchers May Have Solved Origin-of-Life Conundrum,” Science, March 16, 2015, http://www.sciencemag.org/news/2015/03/researchers-may-have-solved-origin-life-conundrum.
- Anne Ju, “Life ‘Not As We Know It’ Possible on Saturn’s Moon Titan,” Cornell Chronicle, February 27, 2015, http://www.news.cornell.edu/stories/2015/02/life-not-we-know-it-possible-saturns-moon-titan.
- Patel et al., “Common Origins of RNA . . . .”
- Charles Darwin, in a letter to J.D. Hooker in 1863, wrote that life “‘appeared’ by some wholly unknown process.” Corresponding again with Hooker in 1871, Darwin speculated about conditions in the prebiotic past that could have resulted in “the first production of a living organism,” writing: “But if (and oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine compound was chemically formed ready to undergo still more complex changes, at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed.” From “Charles Darwin to J. D. Hooker, March 29, 1863,” The Life and Letters of Charles Darwin, Volume 2. Edited by Francis Darwin. New York: Appleton, 1897, pages 202–203; and “To J.D. Hooker 1 February [1871],” Darwin Correspondence Project, https://www.darwinproject.ac.uk/letter/DCP-LETT-7471.xml. Evolutionists, like Darwin, have faith the abiogenesis must have somehow happened but debate where. A more modern evolutionary view has held that life had a marine origin, but some evolutionists have returned the problem of abiogenesis to the warm little pond in the form of geothermal pools. Read more about it in “Primordial Soup Debate” and “Could a Sudsy Pond Bring Forth Life?”
- “Puddy tats” is borrowed from another great story—a quote from the classic Looney Tunes cartoons featuring Tweety Bird and Sylvester the Cat—and from the 1950 song performed by Mel Blanc and the Billy May Orchestra, recently recycled for a new generation by Warner Brothers Pictures as a computer animated short film of the same name, I Tawt I Taw a Puddy Tat.
- K. Ruiz-Mirazo, C. Briones, and A. de la Escosura, “Prebiotic Systems Chemistry: New Perspectives for the Origins of Life,” Chemical Reviews 114, no. 1 (2014): 285–366, doi:10.1021/cr2004844.
- “Not as we know it,” because the proposed “cell” membrane is composed of organic nitrogen compounds “capable of functioning in liquid methane temperatures of 292 degrees below zero” rather than the more water-friendly phospholipid bilayer of Earth’s cells. From Ju, “Life ‘Not As We Know It’ . . . ”
- “Standing Up in the Milky Way,” episode one of Cosmos: A SpaceTime Odyssey, presented by Neil deGrasse Tyson (Santa Fe, NM, Culver City, CA: Cosmos Studios, Fuzzy Door Productions, Santa Fe Studios, 2014).
- Ruiz-Mirazo et al., “Prebiotic Systems Chemistry.”
- Ibid.
- Ibid.
- Ibid.

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis

