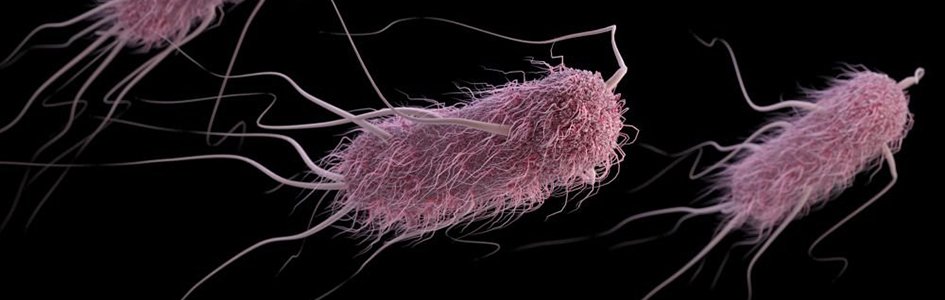
A New Spin on the Bacterial Flagellum: Its Normal Niche and Displacement
Abstract
Antoni van Leeuwenhoek observed individual living cells for the first time in history in 1674. Two years later, he noted microbes with long, thin appendages protruding from globular cells that seemed to provide locomotion, like “little feet” as they moved in drops of water. He gave credit to God in his writings over these new moving wonders. These appendages are now known as flagella (fig. 1), meaning “little whips” (from Latin).
More than 300 years later, Dr. Michael J. Behe used the flagellum and its nanomotor to introduce the concept of “irreducible complexity”—the idea that a structure is so complex that all its parts must initially be present in a suitably functioning manner. The bacterial flagellum is a perfect example of irreducible complexity because all its parts must be present from the start for it to function at all.
According to Darwinian theory, any component that does not offer an advantage to an organism (i.e., does not function) will be lost or discarded. How such a structure as the bacterial flagellum could have evolved in a gradual, step-by-step process as required by classical Darwinian evolution is an insurmountable problem for evolutionists. How a flagellum operates adds an additional level of complexity to the picture.
In the twenty-first century, we know that bacteria are intricately designed but can cause problems if displaced (e.g., urinary tract infections). In the last couple years, bioengineers have taken advantage of microbe motility and “designed displacement” to deliver drugs to diseased body organs.
Keywords: bacterial flagellum, design, displacement, drug delivery
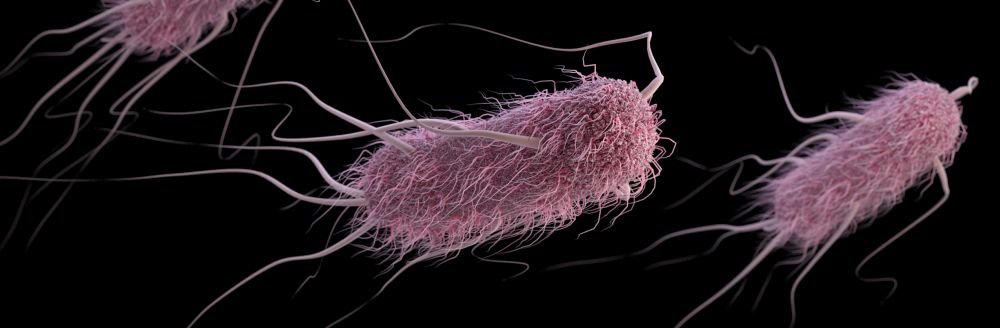
Figure 1. E. coli flagellum. Image credit: CDC.
Introduction
In 1674, Leeuwenhoek, a Christian of the Dutch Reformed faith, was intrigued by “animalcules” (little animals) that he saw in his water using a single-lens microscope. Ones he described as little or “minute eels” were spirillum (probably Spirillum volutans), a large bacterium with flagella. However, flagella weren’t clearly described in detail until 1836 when Christian Ehrenberg saw them on Chromatium okenii. In 1877, Louis Pasteur, a creation microbiologist, saw bacteria in animal blood during his studies of Vibrio septique—later called Clostridium septicum. These bacteria were used in one of his proofs for germ theory. Pasteur called them vibrios (bacteria) with eyelashes (flagella) and was able to photograph stained ones in 1877, using a flagella stain developed earlier that year by Robert Koch. These were bacteria with many flagella (today called peritrichous).
Some bacteria have a single flagellum located at the end of a rod-shaped cell. To move in an opposite direction, a bacterium simply changes the direction the flagellum rotates. Other bacteria have a flagellum at both ends of the cell, using one for going in one direction and the other for going in the opposite direction. A third group of bacteria has many flagella surrounding the cell. They wrap themselves together in a helical bundle at one end of the cell and rotate in unison to move the cell in one direction. To change direction, the flagella unwrap, move to the opposite end of the cell, reform the bundle, and again rotate in a coordinated fashion. The structural complexity and finely tuned coordination of flagella attest to the work of a Master Engineer who designed and created flagella to function in a wonderfully intricate manner (Gillen 2020a).
Fast Facts for Flagella
- Flagella show evidence of creative and intelligent design.
- Some bacteria have only one flagellum, and others have many (fig. 2).
- Bacteria can swim (via flagella) into body locations where they were not intended to be. This is referred to as displacement, and displaced bacteria cause disease.
- Scientists can take advantage of bacteria and protozoans that have flagella and can survive atypical body locations (a designed displacement). This “bioengineered” delivery of microbes may lead to disease treatment or cure (e.g., in cancer or heart treatment) via bacteria or Giardia robots bringing needed cargo (medicine) to the designated site.

Figure 2. Flagellum and base. Image credit: LadyofHats, via Wikimedia Commons.
Fascinating Flagella
Personally, I find flagella fascinating in bacteria and protozoa alike. Under dark-field or phase-contrast microscopy, moving bacteria or protozoa are delightful to watch. They can move fast, spin, turn, reverse directions quickly, swarm, and even do somersaults. Sometimes, you can see the actual thin flagella under phase-contrast or dark-field microscopy. They are very tiny and very fragile, and one must be very careful in handling them. Usually, bacteria have flagella (and active motility) during their early life (16–48 hours). Later, cells often retract them, going into energy-conserving modes. For the genus Bacillus, their energy goes into spore formation, and for Serratia marcescens, pigment production. Once Serratia get a deep red, they don’t move at all.
Flagella can also be stained and seen under bright-field microscopy, but this takes time, patience, and skill. The flagella stain (e.g., Leifson flagella stain) is one of the hardest in microbiology.
The number of flagella vary greatly from one to hundreds per cell. Vibrio cholerae have only one flagellum (monotrichous). Most strains of E. coli have only a few flagella. This bacterium produces 5–10 flagella that are randomly distributed across the cell surface. Serratia marcescens and some strains of Proteus have many flagella (when Serratia marcescens swarms, it can have 100–1000 flagella per swarmer cell).
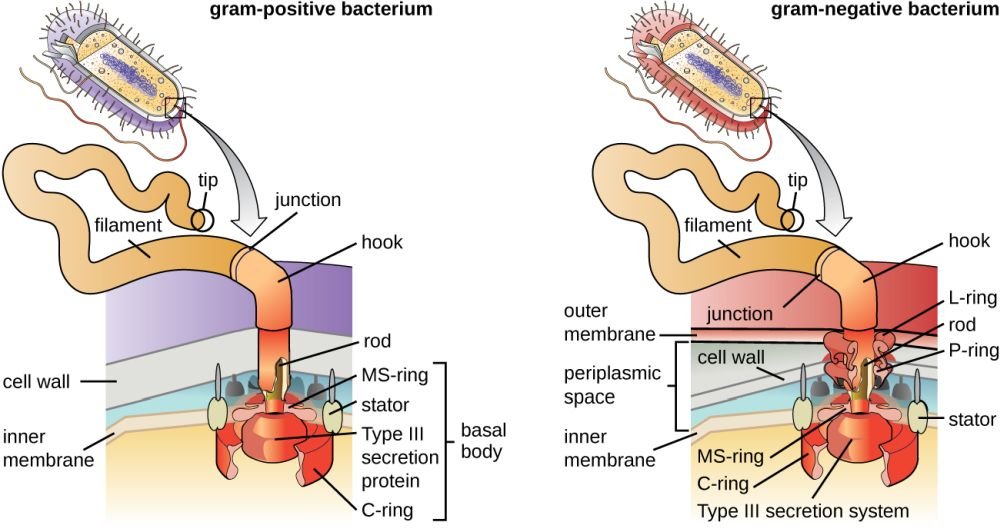
Figure 3. Gram positive vs. negative flagella anatomy. Image credit: CNX OpenStax, via Wikimedia Commons.
Biological Rotary Motor Provides a Rapid Spin through Liquids
Flagella are the Creator’s molecular outboard motors, providing a rapid spin through water, body liquids, and other fluids. Their most interesting aspect is that they are attached to and rotated by tiny, electrical “motors” made of protein. Like an electrical motor, the flagellum contains a rod (drive shaft), a hook (universal joint), L and P rings (bushings/ bearings), S and M rings (rotor), and a C-ring and stud (stator). The flagellar filament (propeller) is attached to the flagellar motor via the hook. The flagellum requires over 40 different proteins and is driven by voltage difference developed across the cell membrane. This motor is one of nature’s best molecular machines! Some scientists have called the bacterial flagellum the “most efficient machine in the universe” with its self-assembly and repair, water-cooled rotary engine, proton motive-force drive system, speeds of up to 17,000 rpm, direction-reversing capability, and hard-wired signal-transduction system with short-term memory.
The sensory and motor mechanisms of the E. coli bacterium consist of several receptors that detect the concentrations of a variety of chemicals. Secondary components extract information from these sensors, which in turn is used as input to a gradient-sensing mechanism, which drives a set of constant torque proton-powered rotary motors that propel helical flagella from 30,000 to 100,000 rpm. This allows the bacterium to move approximately ten body lengths per second. Some have been clocked at up to 100 µm per second, or the equivalent of 50 body lengths per second. As a comparison, bacteria move twice as fast as cheetahs! Generally, bacteria with polar flagella move faster than those with peritrichous (many) flagella.
Again, the complexity of the bacterial flagellum is direct evidence against evolution. In the 1990s, Dr. Michael Behe argued for the intelligent design of the human body. His argument is called the principle of irreducible complexity. To illustrate the complex nature of this principal, one needs to look at flagellar design in driving.
Figure 4a. Bacillus megetarium with its flagella. Image credit: Alan Gillen
Driving by Design—E. coli Swimming Lessons
The more E. coli is studied, the more complex its behavior is revealed to be. Recent observation takes the argument of microbes by design to the next level by providing insight into how E. coli “drive” more orderly than some people. The motion of E. coli is not random; it is directed, ordered, and reminds one of car traffic patterns (or even ant traffic patterns). Harvard researcher, Howard Berg, discovered that E. coli swim on the right side.
In human terms, driving properly to avoid accidents takes driver’s education, intelligence, and practice. It is certainly not by random chance nor accidental. This recent discovery of E. coli driving on the right side—meaning that when placed in narrow, forked tubes, they are more likely to swim up the right-hand fork due to the counterclockwise direction in which the flagella rotate. E. coli can also cooperatively move over surfaces (swimming). Bacteria cells move better on gel surfaces than solid. All this may have clinical implications.
Flagella Engineering
Bacteria may have one flagellum or many in a variety of patterns. Polar flagella extend from the ends of bacteria, whereas peritrichous flagella are distributed randomly over the entire surface (peri means “around,” trichous means “hair”). Bacteria with polar flagella may have anywhere from one flagellum to a tuft of hundreds of flagella at one or both ends of the cell. A few bacteria, called spirochetes, have internalized flagella that lie beneath the cell wall and coil around the cytoplasmic membrane. Bacterial flagella neither flex nor whip like eukaryotic flagella. Instead, they rotate like propellers on a boat. “If a bacterium with a single polar flagellum were held by its flagellum so that the flagellum did not move, then the body of the whole bacterium would rotate. Rotation is accomplished by a basal body and the hook that connects the flagellum to the bacterial cell. The basal body attaches the base of the flagellum to the cytoplasmic membrane and cell wall and acts as a motor to turn the flagellum. The hook transfers the rotation from the basal body to the external flagellar filament” (Gillen 2020a).
Flagella rotate counterclockwise to propel bacteria forward, driven by chemotaxis, which is the movement of bacteria in response to chemicals in the environment. These chemicals can be used as energy sources, and bacteria have receptors on their surface to detect them. When such a molecule interacts with the receptor, a signal is sent to the basal body, the flagellar motor starts, and the bacterium moves toward the energy source.
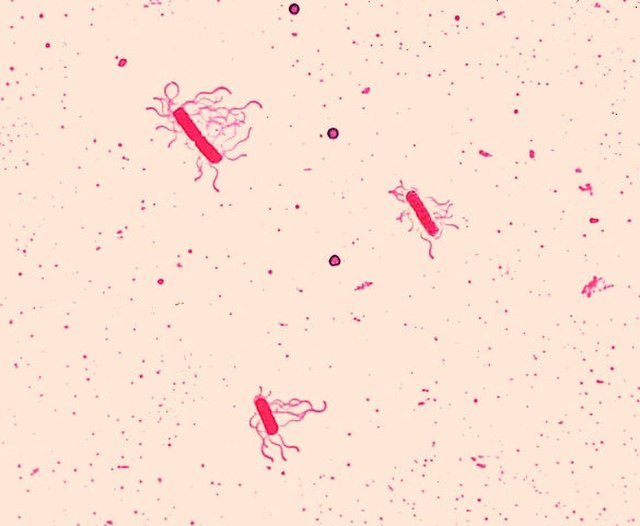
Figure 4b. Bacillus species, Bacillus cereus, Leifson flagella stain. Image credit: CDC/Dr. William Clark
Bacteria with a single polar flagellum move simply back and forth. They move forward by rotating their flagellum counterclockwise, and backward by rotating their flagellum clockwise. Bacteria with multiple flagella move via the synchronized action of all the flagella. These bacteria show an overall pattern of movement consisting of a series of “runs” (or “swims”) and “tumbles.” During a run, all the flagella work together as a functional bundle and rotate synchronously in a counterclockwise direction to propel the bacterium toward the energy source. During tumbles, the flagellar bundles disassemble. The time spent in runs determines how fast the bacterium moves in a specific direction and depends on the concentration of the energy source. The greater the concentration of molecules of the energy source, the more interaction with the receptors, the more rotation, and the farther the bacterium moves. As the concentration decreases, there is less interaction with the receptors, flagella are engaged less, and the bacterium does not move as far. Instead, the bacterium tumbles more often. During a tumble, when flagella turn clockwise, the bacterium ceases forward motion and “jiggles.” Once the tumble is completed, the bacterium moves randomly away from the site of the tumble. So the more a bacterium tumbles, the greater the chance it will not move in a definite direction.
A New Spin—Displacement
E. coli is normally an intestinal, commensal bacterium that can adapt to new environments in the body. E. coli swims faster in the urinary tract than in the gut due to less resistance and viscosity of fluids. According to Harvard biologist Howard Berg, “E. coli lives a life of luxury in the lower intestines of warm-blooded animals, including humans. Once expelled, it lives a life of penury and hazard in water, sediment, and soil. E. coli is a minor constituent of the human gut. A typical stool contains as many as 1011 (100 billion) bacteria per cubic centimeter (cm3). Up to 109 (1 billion) of these are E. coli. Most of the other bacteria are strictly anaerobic, and thus unable to live in the presence of oxygen outside of the body. Cells of E. coli can live with or without oxygen, and thus survive until they find another host or another part of the body . . . . If fed well, however, it grows to a density like that of its siblings there, to some 109 cells per cm3: the population of India in a spoonful” (Berg 2004). Once out of this normal microhabitat and niche, it can adapt to most places in the body if given the right nutrients.

Figure 5. Reagent strips for urinalysis. Image credit: Alan Gillen.
A urinary tract infection (UTI) is an infection involving any part of the urinary system—including urethra, bladder, ureters, and kidney—and occurs when germs (bacteria) invade the urinary tract. UTIs are the most common type of healthcare-associated infection reported to the CDC, with 6–8 million UTI cases diagnosed each year in the United States. According to Karami et al. (2020), 80 to 90% of UTIs are caused by E. coli. For the most part, E. coli lives harmlessly in the gut, but it can cause problems if it enters the urinary system.

Figure 6. E. coli nitrate broth urine stick. Image credit: Alan Gillen.
A different scenario for displacement is when Proteus mirabilis enters the bloodstream through wounds. (The most frequent cases are among the elderly with declining immune systems.) This happens with contact between a wound or sore and an infected surface. The bacteria then induce an inflammatory response that can cause sepsis and urosepsis and damage to the kidney and ureters. On rare occasions, P. mirabilis can also colonize the lungs, perhaps by infected hospital breathing equipment, causing pneumonia. In each case, the bacteria are “swimming” through the blood, lymph, or body fluids via flagella.
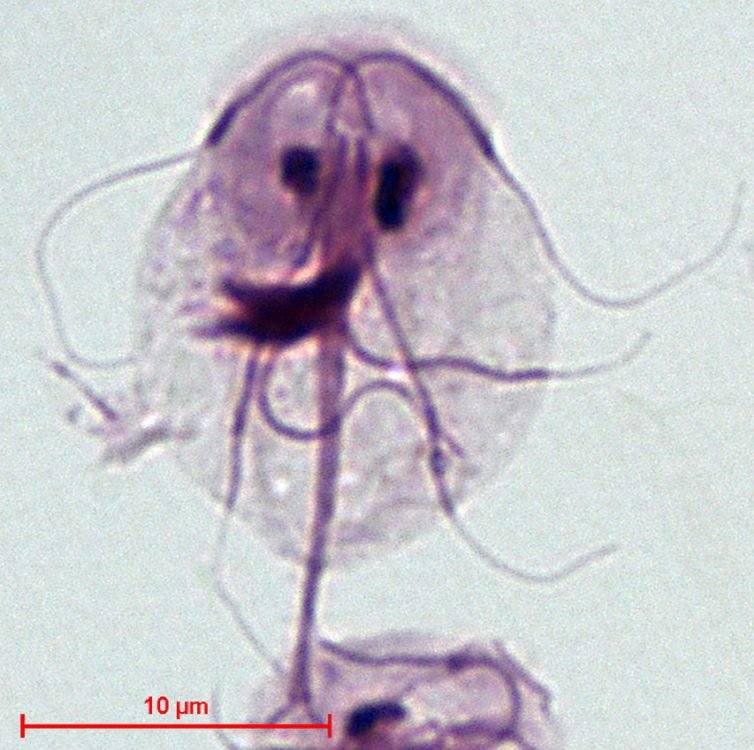
Figure 7. Giardia intestinalis. Image credit: Stefan Walkowski, via Wikimedia Commons.
Medicines from Bioengineered Bacteria and Giardia Offer Promise
The essence of this approach is using “bugs” (via motorized microbes) to deliver drugs. We can use bacteria or Giardia’s swimming ability (via flagella) to send them to internal organs and deposit drugs.
E. coli’s flagella are the most studied, but magnetotactic bacteria are also promising. These species of soil bacteria synthesize iron oxide nanoparticles called magnetosomes as “compasses” that allow them to navigate in the earth’s magnetic field to find optimum conditions for growth and survival. These bacteria with microscopic magnets are perfectly shaped and ideally suited to the microscopic packages we need to target deep cancers (like breast and prostate cancer) (McKie 2022). Magnetotactic bacteria also have hyperdrive-equipped flagella and are reported to swim ten times faster than E. coli because of their gear-driven, seven-engine, magnetic-guided flagellar bundles that can accelerate from 0 to 300 micrometers (µm) in one second. With this capability, they could deliver medicine quickly.
Of another example of bacteria flagella delivery systems, GEN 2020 states, “A biohybrid microswimmer—a genetically engineered bacterium studded with nanoerythrosomes—can be loaded with molecular cargo, injected into the body, and sent on a delivery mission. For example, the microswimmer could propel itself through viscous environments and tissue cells to dispense drugs at a tumor site. To get where it needs to go, the microswimmer could home in on a signal of some kind. A chemical signal could allow microswimmer dispatchers to take advantage, however passively, of a bacterium’s natural sensing capabilities. Alternatively, magnetic or sound signals could allow for a more active, hands-on approach. That is, microswimmer movements could be subjected to remote control.” In this example, nanoerythrosomes were attached to the bacterial membrane using a biological bond. This process preserved two important red blood cell membrane proteins: one needed to attach the nanoerythrosomes, and one to prevent macrophage uptake.
One bioengineered E. coli strain served as a bioactuator (an organism that produces a motion by converting energy and signals going into the system) performing the mechanical work of propelling through the body using flagellar rotation. The swimming capabilities of the bacteria were assessed using a custom-built algorithm and videos to document their performance. These biohybrid microswimmers performed at speeds 40% faster than other non-engineered E. coli-powered microparticles-based biohybrid microswimmers. They “demonstrated a reduced immune response due to the nanoscale size of the nanoerythrosomes and adjustments to the density of coverage of nanoerythrosomes on the bacterial membrane. These biohybrid swimmers could deliver drugs faster, due to their swimming speed, and encounter less immune response, due to their composition” (Genetic Engineering and Biotechnology News 2020).
Giardia Microrobots
Giardia are protozoans with eight flagella. As Georgia Purdon (2012) previously reported, bioengineered Giardia robots could possibly be used as nanomachines to deliver drugs to places like the kidneys. If these microrobots can imitate the swimming abilities of wild Giardia, doctors could use them for drug delivery and breaking up kidney stones. The technology has promise but still needs more work to become useful in a hospital setting.
Summary and Conclusions
Motile microbes are fascinating. Both bacteria and protozoa with flagella show evidence of design. Some of the first electron microscope images of the flagellum, presumably from Berg’s Harvard lab, spoke for themselves in this regard. Berg was probably the foremost authority on E. coli in motion, illustrating the flagellum of bacteria. His work also inspired Dr. Richard Bliss, who spoke about the intricate creation of the bacterial flagellum ten years before Michael Behe.
Flagellated bacteria and protozoa in their designed host and place (as microbiome) can benefit their host, themselves, and nature. However, due to their mobility and adaption they can travel other places in their host—human or animal. Displaced from their normal niche and microhabitat, they can cause great distress and disease to their host. The most notable example with bacteria is through entering the urinary system and causing UTIs and kidney infections. And Giardia, in particular, can cause significant diarrhea and in immunocompromised children could lead to death.
Some bacteria are self-propelled via flagella equipped with magnetic crystals to navigate the body. Magnetostatic bacteria live in murky water on little oxygen, and their crystals align with the magnetic field of the earth to navigate to good environments for orientation.
Flagellated bacteria preloaded with cancer drugs offer promise in medicine. Magnetosomes carry the iron crystals, and these crystals can naturally navigate to the right body organs. But it takes the flagellum to propel the bacteria loaded with drugs and magnets to cancerous organs. Motility via flagella is key in bacterial therapy, since bacteria can actively swim and penetrate deep into tumor tissue. Tumors display irregular and chaotic vasculature, leading to areas with low oxygen concentration and nutrient limitation. Such hypoxic (low oxygen) regions are a perfect niche for anaerobic (no oxygen) and microaerophilic (low oxygen) bacteria to perform selective colonization. The mechanism behind this bacterial therapy is still not well understood, but there is evidence indicating that bacteria could perform direct oncolysis (cancer cell destruction) and stimulate the immune system. The amazing designs given by the Creator give bioengineers ideas to make their own flagellated “nanofactories” to function in drug delivery and to help mitigate the effects of mankind’s sin-caused curse.
These discoveries may expand to use other flagellated bacteria and protozoans as well as lead to the design of bio-inspired, swimming micro-robots for nanomedicine, with site-specified and controlled drug delivery that is less invasive than surgical procedures. Bioengineers are trying to think like the Master Bioengineer in bringing healing and restoration. Thinking God’s thoughts after him (Psalm 139:17) could bring both new revelation in science and help in restoration of a diseased body in a fallen world.
References
Michael J. Behe, Darwin’s Black Box: The Biochemical Challenge to Evolution, New York: Free Press, 2006.
Michael J. Behe, A Mousetrap for Darwin: Michael J. Behe Answers His Critics, Seattle, WA: Discovery Institute, 2020.
Howard C. Berg, E. coli in Motion, New York: Springer, 2004.
“Urinary Tract Infection,” Antibiotic Prescribing and Use, CDC, last reviewed October 6, 2021, archived December 6, 2023, at https://web.archive.org/web/20231206222459/https://www.cdc.gov/antibiotic-use/uti.html.
Nahid Karami, Anna Lindblom, Shora Yazdanshenas, Viktoria Lindén, and Christina Åhrén, “Recurrence of Urinary Tract Infections with Extended-Spectrum β-Lactamase-Producing Escherichia coli Caused by Homologous Strains Among Which Clone ST131-O25b Is Dominant,” Journal of Global Antimicrobial Resistance 22 (2020): 126–132, https://doi.org/10.1016/j.jgar.2020.01.024.
Alan L. Gillen, The Genesis of Germs: Disease and the Coming Plagues in a Fallen World, rev. ed., Green Forest, AR: Master Books, 2020.
Alan L. Gillen, Body by Design: Fearfully & Wonderfully Made, Green Forest, AR: Master Books, 2020.
Alan L. Gillen and Frank Sherwin, “The Design of Giardia and the Genesis of Giardiasis,” Answers In-Depth, July 19, 2017, https://answersingenesis.org/biology/microbiology/design-giardia-and-genesis-giardiasis/.
Scott C. Lenaghan et al., “High-Speed Microscopic Imaging of Flagella Motility and Swimming in Giardia lamblia Trophozoites,” Proceedings of the National Academy of Sciences 108 (34): E550–558, https://doi.org/10.1073/pnas.1106904108.
Georgia Purdom, “Magnificent Motors: God Invented It First,” Answers Magazine, January 1, 2012, https://answersingenesis.org/biology/microbiology/magnificent-motors/.
“Drug Delivery via Biohybrid Microswimmers a Flagellum Lash Closer,” Genetic Engineering and Biotechnology News, April 8, 2020. https://www.genengnews.com/topics/drug-discovery/drug-delivery-via-biohybrid-microswimmers-a-flagellum-lash-closer/.
Robin McKie, “Magnets Made by Soil Bacteria Offer Hope for Breast and Prostate Cancer,” The Guardian, May 8, 2022, https://www.theguardian.com/science/2022/may/08/magnets-made-by-soil-bacteria-offer-hope-for-breast-and-prostate-cancer.
Answers in Depth
2022 Volume 17
Answers in Depth explores the biblical worldview in addressing modern scientific research, history, current events, popular media, theology, and much more.
Browse VolumeAbout the author: Dr. Alan L. Gillen is a professor of biology at Liberty University.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


