Survey of Microbial Composition and Mechanisms of Living Stromatolites of the Bahamas and Australia: Developing Criteria to Determine the Biogenicity of Fossil Stromatolites
Abstract
A stromatolite is typically defined as a laminated and lithified structure that is the result of microbial activity over the course of time. Fossil stromatolites are relatively abundant; however, modern living stromatolites are rare. Two well-studied examples of living stromatolites include those found in the Exuma Cays of the Bahamas and Shark Bay in Australia. Depending on dominant chemical reactions by bacteria and environmental conditions, accretion and lithification of the stromatolite occurs at intervals. Each layer or lamina of a stromatolite represents a former surface mat of bacteria. As long as cyanobacteria (or other phototrophs) colonize the top surface of the stromatolite, growth is likely to continue. Understanding microbial composition and mechanisms of living stromatolites is crucial to determining the biogenicity of fossil stromatolites. Although there is a paucity of fossilized bacteria in fossil stromatolites, their structural features closely resemble those of living stromatolites. A set of criteria from the study of living and fossil stromatolites has been developed to aid determination of the biogenicity of fossil stromatolites. It was concluded that there is now sufficient evidence for the biogenicity of many stromatolites, even as early as 3.5 Ga, so these need to be understood within the biblical framework of earth history. Discernment of genuine stromatolites in the geologic record may help determine boundaries between Creation Week, pre-Flood and Flood strata. In addition, understanding how various living stromatolites form in different environments provides insight into the pre-Flood environments in which fossil stromatolites grew.
Keywords: Living stromatolites, fossil stromatolites, biogenicity, cyanobacteria, endolithic, heterotrophic, lithification, lamination, calcium carbonate, accretion, precipitation, mineralization, organomineralization, Exuma Cays, Hamelin Pool, extrapolymeric substance (EPS), sulfate reducing bacteria (SRB), Precambrian strata, Flood strata, microfossils, Creation Week, pre-Flood era
Introduction
Stromatolite definitions, although varied, typically refer to an organosedimentary laminated and lithified structure produced by sediment trapping, binding, and/or precipitation as a result of the growth and metabolic activity of microorganisms, principally cyanobacteria and heterotrophic bacteria over the course of time (Chivas et al. 1990; Reid et al. 1995; Visscher et al. 1998; Papineau et al., 2005). The word stromatolite comes from the Greek stromat meaning to spread out and lithos meaning stone (Riding, 2000). Fossil stromatolites, though sparse in the geologic record, are nevertheless relatively abundant in terms of the number of occurrences (Semikhatov & Raaben 1996; Grotzinger & Knoll 1999; Schopf, 2004); however, modern living stromatolites are rare.
Although by definition stromatolites are biogenic, some scientists have questioned whether these structures are exclusively biogenic and might instead be the result of abiogenic mechanisms (Lowe 1994; Hladil, 2005; Brasier et al., 2006). Understanding microbial composition and mechanisms of living stromatolites is crucial to determining the biogenicity of fossil stromatolites. Fossil stromatolites have been found mainly in Archean (3500–2500 Ma) and Proterozoic (2500–541 Ma) strata, with comparatively minuscule numbers in Phanerozoic (541 Ma–Present) strata (Gradstein et al., 2012). Geologists and paleontologists have questioned the biogenicity of the oldest Archean stromatolites due to the difficulties of identifying signatures of life, such as microfossils, in rock layers that are dated to 3.5 billion years old (Awramik & Grey, 2005). For scientists believing in an earth that is 4.5 billion years old, a biogenic origin for early Archean stromatolites is problematic because it leaves only a scant one billion years for the evolution of cyanobacteria and their major metabolic process of photosynthesis from non-life (Awramik & Grey, 2005).
The biogenicity of fossil stromatolites is also problematic for creationists. Stromatolites are believed to form over extended periods of time from hundreds to thousands of years. Archean strata likely represent Creation Week rocks (Snelling, 2009), so how can stromatolites that evidently take long periods of time to form be present in rocks that were created in less than a week? Proterozoic strata likely represent late Creation Week and pre-Flood era rocks (Snelling, 2009) and would have formed over a period of approximately 1650 years. The biogenicity of Proterozoic stromatolites is typically not questioned even within the secular scientific community (Hofmann et al. 1999). Structurally there seems to be little difference between Archean and Proterozoic stromatolites (Awarmik & Grey, 2005), leading to the potential conclusion that if Proterozoic stromatolites are biogenic, then so are Archean stromatolites.
Fossil stromatolites in Phanerozoic strata are also potentially problematic for creationists because those strata are believed to have formed very rapidly during Noah’s Flood (Snelling, 2009). Again, how could structures that appear to take long periods of time to grow form over the course of a year? Could the nature and pattern of occurrence of fossil stromatolites be useful in determining the pre-Flood/Flood/post-Flood boundaries?
Studying living stromatolites is absolutely essential to determining the biogenicity of fossil stromatolites and understanding how such structures form and grow. The microstructure of living stromatolites closely resembles that of fossil stromatolites (although there is a paucity of fossilized bacteria in fossil stromatolites) (Reid et al. 1995; Visscher et al. 1998). Thus living stromatolites are potentially a good model for developing criteria to determine the biogenicity of fossil stromatolites.
The purpose of this study is to develop a set of criteria from the study of living and genuine fossil stromatolites to aid determination of the biogenicity of other fossil stromatolites. Discernment of genuine stromatolites in the geologic record may help determine boundaries between Creation Week, pre-Flood and Flood strata. In addition, understanding how various living stromatolites form in different environments provides insights into the pre-Flood environments in which fossil stromatolites grew.
Survey of Living Stromatolites
Stromatolites consist of “guilds” of bacteria that perform different functions, such that the end metabolic products produced by one bacterial guild provide the starting metabolic products for a different guild (Visscher & Stolz, 2005; Dupraz et al., 2009). This distribution of functions benefits the microbial community and effectively builds the stromatolite. Microbial composition varies, but typically consists of three major bacterial types—cyanobacteria, heterotrophic bacteria, and endolithic cyanobacteria (Baumgartner et al., 2009; Papineau et al., 2005; Goh et al., 2009; Allen et al., 2009). Cyanobacteria are active in photosynthesis and play a major role in the overall growth of the stromatolite. Metabolic activities of heterotrophic bacteria create conditions (in conjunction with environmental factors) that result in the precipitation and mineralization of calcium carbonate leading to lithification of the stromatolite. Endolithic cyanobacteria bore through sand grains of the stromatolite welding together grains and stabilizing the structure of the stromatolite.
Lithification (the process of sediments becoming solid rock) results directly from microbial activity performed mainly by heterotrophic bacteria (in conjunction with environmental factors) (Dupraz & Visscher, 2005). However, cyanobacteria are responsible for accretion (addition) of sediment through the trapping and binding of sand grains (Dupraz & Visscher, 2005). Microbial activity consists of a complex set of chemical reactions that result in both the dissolution and precipitation of calcium carbonate (Dupraz & Visscher, 2005). When metabolic processes result in net precipitation/mineralization of calcium carbonate, lithification occurs (Dupraz & Visscher, 2005).
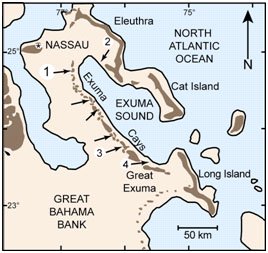
Figure 1. Map of Exuma Cays, Bahamas. Arrows point to locations of stromatolites. Reprinted from Figure 1, Visscher et al., 1998.

Figure 2. Columnar Bahamian stromatolites. Size range of stromatolites in photo is 0.25 to 0.5 m. Reprinted from Plate 4—1d, Reid et al., 1995.
Depending on dominant chemical reactions by bacteria and environmental factors, accretion and lithification of the stromatolite occurs at intervals (Dupraz et al., 2009). Each layer or lamina of a stromatolite represents a former surface mat of bacteria (Reid et al., 2000). As long as cyanobacteria (or other phototrophs) colonize the top surface of the stromatolite, growth is likely to continue (Reid et al., 2000). Although several studies have been done to estimate the growth rate of stromatolites, no consensus has been reached. However, most living stromatolites are considered to be no more than several thousand years old (Macintyre et al. 1996; Jahnert & Collins, 2012).
For the purpose of developing criteria to determine the biogenicity of fossil stromatolites, we will examine two of the most widely studied living stromatolites—those in the Exuma Cays, Bahamas and Hamelin Pool in Shark Bay, Australia.
Exuma Cays, Bahamas
The Exuma Cays consist of more than 360 islands or cays. Stromatolites grow in an open marine, normal saline environment in subtidal and intertidal areas (Reid et al. 1995) of fringing reefs of the Exuma Cays (Visscher et al. 1998) (Figure 1).
The dominant morphology of Bahamian stromatolites is columnar with heights ranging from a few centimeters to 2.5 meters (Andres & Reid, 2006) (Figure 2).
Cyanobacteria are typically thought of as the main microbial contributor to Bahamian stromatolite formation. However, a rich diversity of microbes is necessary for stromatolite growth and lithification.
Stromatolites alternate between periods of active sediment accretion and sediment hiatus (no accretion and lithification) (Reid et al., 2000). Three types of surface mats with varying microbial compositions are associated with stromatolites. Type 1 is associated with periods of sediment accretion and Types 2 and 3 are associated with sediment hiatus (Reid et al., 2000). The dominant bacterial species discovered in Bahamian stromatolites belong to the following groups: Cyanobacteria, Bacteroidetes (heterotrophic bacteria), Proteobacteria (heterotrophic bacteria) and Planctomycetes (heterotrophic bacteria) (Baumgartner et al., 2009) (Table 1).
| Type 1 | Type 2 | Type 3 | Water | |
|---|---|---|---|---|
| Bacteria | 95.2 | 99.2 | 95.7 | 100.0 |
| Proteobacteria | 41.3 | 57.4 | 52.8 | 66.2 |
| Alphaproteobacteria | 32.1a | 49.0 | 41.8 | 56.3 |
| Deltaproteobacteria | 3.2 | 6.4 | 6.0 | |
| Gammaproteobacteria | 6.0 | 2.0 | 5.1 | 9.9 |
| Planctomycetes | 14.3 | 11.2 | 13.4 | 1.4b |
| Cyanobacteria | 10.3 | 14.3 | 9.9 | 14.1 |
| Bacteroidetes | 17.5 | 7.6c | 6.0d | 16.9 |
| Chloroflexi | 2.4 | 2.4 | 5.7 | 1.4 |
| Spirochaetes | 2.4 | 0.8 | 2.8 | |
| Verrucomicrobia | 2.4 | 2.4 | 0.9 | |
| Actinobacteria | 2.4 | 0.3 | ||
| Firmicutes | 0.4 | 1.2 | 0.3 | |
| Chlorobi | 0.9 | |||
| Deferribacteres | 0.8 | |||
| Other Bacteria | 2.0 | 1.2 | 2.8 | |
| Archaea total | .8 | 1.4 | ||
| Eukarya total | 4.4 | 2.8 | ||
Foster et al. (2009) analyzed cyanobacteria from a variety of stromatolite types and showed that several of the filamentous cyanobacteria identified exhibited gliding motility in response to light (phototaxis). This characteristic of cyanobacteria is vital following burial of stromatolites by sand or other sediment as the bacteria can move to the surface to capture sunlight necessary for photosynthesis and continued growth of the stromatolite.
Type 1 mats are typically caramel colored or light green and are 0.5–1 mm thick (Stolz et al., 2009). These mats exhibit the least microbial diversity with filamentous cyanobacteria as the major microbial component. Schizothrix gebeleinii was thought to be the dominant cyanobacteria in Bahamian stromatolites (from morphological studies); however, genetic analysis of DNA libraries derived from Bahamian stromatolites showed no Schizothrix DNA was present (Baumgartner et al., 2009). These stromatolites appear to harbor many novel species of cyanobacteria based on sequence analyses (Baumgartner et al., 2009).
Type 2 mats are white or gray-green, mucilaginous (due to abundant amounts of extracellular polymeric substance (EPS) produced by bacteria), somewhat brittle and flaky (due to thin micritic crusts consisting of calcium carbonate crystals <4 μm in diameter), and are 20–100 μm thick (Stolz et al., 2009). They harbor a wide variety of heterotrophic bacteria (Baumgartner et al., 2009).
Type 3 mats are white or gray-green, crusty and hard (due to micritic crust formation) and are 50–100 μm thick (Stolz et al., 2009). The major microbial component of these mats is an endolithic coccoid cyanobacteria recognized by morphological studies to be Solentia sp. and Hyella sp. (Stolz et al., 2009).
As mentioned previously, different mat types are associated with periods of sediment accretion (Type 1) and hiatal intervals (Types 2 and 3). Mat types have also been shown to alternate with the seasons. Type 1 is common year round, Type 2 is nearly absent in spring and winter but common in summer and fall and Type 3 is present year round but more common in spring and winter (Stolz et al., 2009).
The influence of these environmental factors for mat types, and thus formation of stromatolites, has implications to rapidly changing and fluctuating conditions that were present during Creation Week and during and after the Flood. The effects of perturbations, such as those mimicking Flood conditions, are potentially testable since stromatolites can be grown in a lab environment (Havemann & Foster, 2008).
Hamelin Pool, Shark Bay, Australia
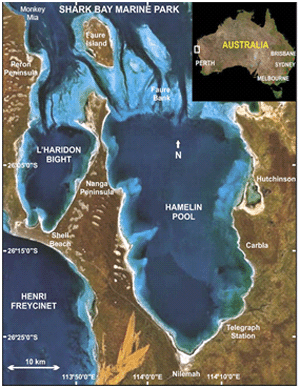
Figure 3. Map of Hamelin Pool, Shark Bay, Australia. Stromatolites are found in subtidal and intertidal areas near the borders of the pool. Reprinted from Figure 1, Jahnert & Collins, 2012.
Hamelin Pool is a shallow, hypersaline embayment that is separated by a grass bank from the rest of Shark Bay on the Western Australian coast (Reid et al., 2003) (Figure 3). High evaporation levels and reduced flow of seawater into Hamelin Pool make the salinity twice that of normal seawater (Goh et al., 2006). Stromatolites are found in both intertidal and subtidal areas covering approximately 100 km of shoreline (Reid et al., 2003).

Figure 4. Hamelin Pool stromatolites at low tide. Stromatolites in photo are approximately 40 cm high. Reprinted from Plate 45, Figure 1a, Reid et al., 2003.
They have a wide range of morphologies including columnar, club, spheroidal, domal, and ellipsoidal (Papineau et al., 2005; Jahnert & Collins, 2012) (Figure 4). Maximum height is approximately 1.5 m (Jahnert & Collins, 2012).
Microbial composition of Hamelin Pool stromatolites shares many similarities to that of Bahamian stromatolites despite differences in their external environments. Genetic analyses of microbial diversity in Hamelin Pool stromatolites have been conducted comparing different morphological types (macroscopic structure) of stromatolites versus microbial diversity among individual mat types as with Bahamian stromatolites (Allen et al., 2009). The reason is that Hamelin Pool stromatolites do not consist of specific mat types associated with dominant bacterial groups (Jahnert & Collins, 2012) (Figure 5).

Figure 5. Proposed sequence of Hamelin Pool stromatolite formation (A=pioneer community to D=climax community). Despite the lack of localization of particular bacterial populations in Hamelin Pool stromatolites, many similarities to Bahamian stromatolites can be found. A is similar in microbial composition to a Type 1 mat in Bahamian stromatolites due to the abundance of cyanobacteria on the top surface. B and C are similar to a Type 2 mat in Bahamian stromatolites due to micritic crust formation. D is similar to a Type 3 mat in Bahamian stromatolites due to the formation of aragonite infilled boreholes. Reprinted from Figure 16 A–D, Jahnert & Collins, 2012.
However, macroscopically Hamelin Pool stromatolites still have a laminar appearance.
The dominant bacterial species discovered in these stromatolites belong to the following groups: Proteobacteria, Planctomycetes, Actinobacteria (heterotrophic bacteria), and Bacteroidetes (Papineau et al., 2005; Goh et al., 2009; Allen et al., 2009). Most of the groups are the same as those found in Bahamian stromatolites, although individual species may differ between them (Table 1 and Figure 6). For example, Microcoleus sp. is the dominant cyanobacteria in contrast to Schizothrix and/or novel cyanobacteria that are dominant in Bahamian stromatolites (Allen et al., 2009). One of the main microbial contributors appears to be anoxygenic phototrophs like those belonging to the Proteobacteria group (Papineau et al., 2005). Anoxygenic photosynthesis does not result in the production of oxygen and is likely beneficial for the metabolism of heterotrophic bacteria that also play a major role in the formation of stromatolites. Halophilic archaea are also found in Hamelin Pool stromatolites and comprise about 10% of the microbial community (Goh et al., 2009). As with Bahamian stromatolites, Hamelin Pool stromatolites harbor many novel species of bacteria. For example, a novel halophilic archaea, Halococcus hamelinensis, has been identified (Goh et al., 2006).
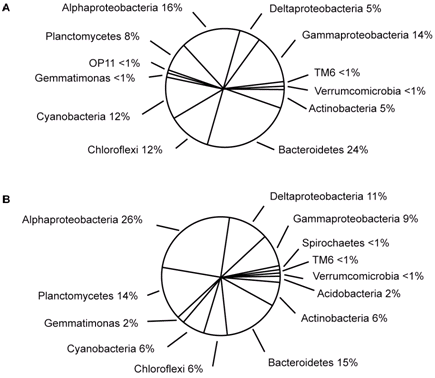
Figure 6. Microbial diversity of Hamelin Pool stromatolites. A (pustular mat) and B (smooth mat) represent different morphological types of stromatolites. Reprinted from Figure 3, Allen et al., 2009.
Role of Microbial Metabolic Activity in the Formation of Stromatolites
Metabolic activities will be discussed in relation to Bahamian stromatolites as extensive research has been published in this area. Microbial composition of both Bahamian and Hamelin Pool stromatolites is similar, thus metabolic activities of the microorganisms are also likely similar even if the organization of the microorganisms is different.
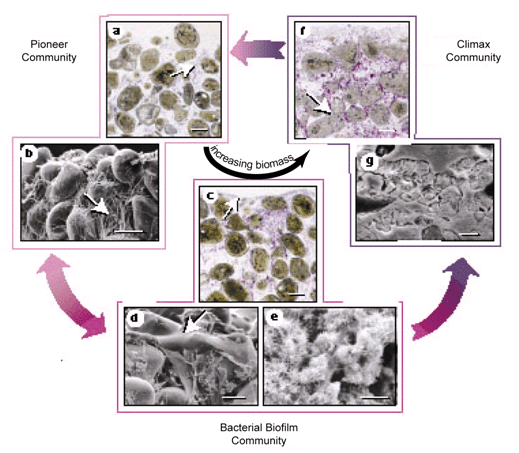
Figure 7. Cycling of bacterial communities in Bahamian stromatolites resulting in the formation of Type 1 (a–b), 2 (c–e), and 3 (f–g) mats resulting in PML and stabilization of the stromatolite over time. Reprinted from Figure 2, Reid et al., 2000.
Metabolic activities of microorganisms in the three different mat types result in growth of the stromatolite through the processes of precipitation, mineralization, and subsequent lithification (abbreviated PML for the sake of brevity). (Environmental factors also play a role and these will be discussed below.) A cycling process of mat types occurs at the surface of the stromatolite in relation to seasons and sedimentation rates. Type 1 mats represent pioneer communities that colonize the stromatolite surface during periods of sediment accretion (Reid et al., 2000) (Figures 7 and 8). Type 2 mats represent a biofilm community that colonizes the stromatolite surface during sediment hiatus (Reid et al., 2000) (Figures 7 and 8). PML in Type 2 mats results in the formation of micritic crusts.
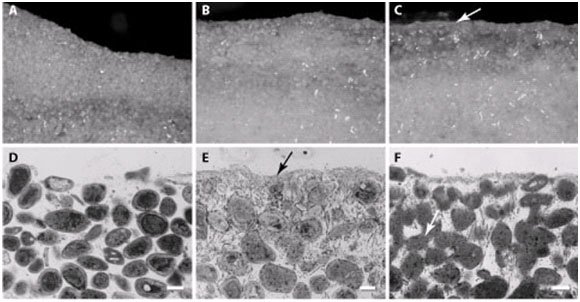
Figure 8. Cross section of Type 1 mat (A, B), Type 2 mat (C, D), and Type 3 mat (E, F). A, C, and E are stereomicroscope images and B, D, and F are light microscope images. Arrow in C is pointing to the micritized crust formed as a result of the metabolic activity of abundant heterotrophic bacteria in this mat type. Arrow in E is pointing to fused sediment grains due to the boring activity of endolithic bacteria forming boreholes that are subsequently infilled. Reprinted from Figure 1 a–f, Baumgartner et al., 2009
Prolonged periods of sediment hiatus result in Type 3 mats representing a climax community that colonizes the stromatolite surface (Reid et al., 2000) (Figures 7 and 8). PML in Type 3 mats results from the welding or fusing together of sand grains that help stabilize the stromatolite. When sedimentation increases again and a Type 1 mat dominates the surface, Type 2 and 3 mats (now below the surface) are still metabolically active and continue to lithify, and thus stabilize the stromatolite (Visscher et al. 1998).

Figure 9. Cross-section of Bahamian stromatolite showing laminations. C brackets a Type 1 mat on the surface of the stromatolite. M with arrow points to micritic crust associated with a former surface Type 2 mat. F brackets a former surface Type 3 mat with endolithic bacteria. Reprinted from Figure 2 A and B, Visscher & Stolz, 2005.
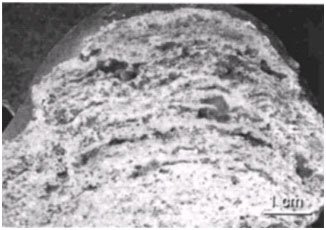
Figure 10. Cross-section of Hamelin Pool stromatolite showing laminations. Reprinted from Plate 52, Figure 1a, Reid et al., 2003.
Over time this cycling of surface mats gives the stromatolite a laminated appearance, with each lamina representing a former surface mat (this is also true for Hamelin Pool stromatolites) (Figures 9 and 10). Both Bahamian and Hamelin Pool stromatolites are believed to grow less than a millimeter a year (Jahnert & Collins, 2012). If the periodicity of the lamination could be determined then age estimates could be made for living stromatolites. Although the oldest living stromatolites are not considered to be more than a few thousand years old (Macintyre et al. 1996; Chivas et al. 1990), the periodicity of lamina formation is highly variable. This also lessens the possibility of determining the time period necessary to form ancient/fossil stromatolites. However, it seems possible that stromatolite formation could occur rapidly under certain environmental conditions that may lend support to their rapid formation during Creation Week and the Flood.
Photosynthesis and aerobic respiration are the dominant metabolic activities of Type 1 mats (Visscher & Stolz, 2005). Cyanobacteria perform photosynthesis and actively fix carbon dioxide using light energy with the end result being the formation of various sugars. These sugars (polysaccharides) are secreted in copious amounts from the bacteria and form the extrapolymeric substance (EPS) (Riding, 2000). EPS composes the mucilaginous sheaths surrounding individual cyanobacteria. EPS is mucus-like or “sticky” and aids in bacterial attachment to substrates, protection, and nutrient absorption (Riding, 2000). EPS also traps calcium ions and sediments. Photosynthesis and concomitant geochemical reactions in the EPS lead to net calcium carbonate precipitation (Visscher & Stolz, 2005) (Figure 11).
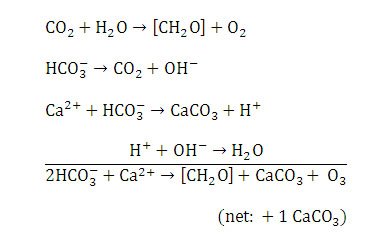
Figure 11. Chemical reactions resulting in calcium carbonate precipitation in Type 1 mats. Reprinted from Reaction 1, Visscher et al., 1998.
Heterotrophic bacteria perform aerobic respiration and metabolize some of the EPS formed by the cyanobacteria (Visscher & Stolz 2005). Metabolism of EPS and concomitant geochemical reactions cause dissolution of the calcium carbonate (Visscher & Stolz, 2005) (Figure 12). There is little to no net calcium carbonate precipitation in Type 1 mats due to the balance of calcium carbonate precipitation and dissolution (Visscher & Stolz, 2005).
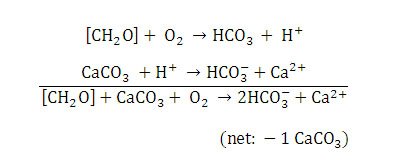
Figure 12. Chemical reactions resulting in calcium carbonate dissolution in Type 1 mats. Reprinted from Reaction 2, Visscher et al., 1998.
Sulfate reduction is the dominant metabolic activity of heterotrophic bacteria in Type 2 mats. Sulfate reducing bacteria (SRB) quickly degrade the copious amounts of EPS formed by cyanobacteria (in both Type 1 and 2 mats) as their carbon and energy source.
Since Type 2 mats usually form on top of Type 1 mats during a sediment hiatus this rich resource is readily available to them. EPS degradation also releases calcium (Dupraz & Visscher, 2005). Metabolism of EPS by SRB and concomitant geochemical reactions cause net precipitation of calcium carbonate (Dupraz et al., 2009) (Figure 13). Visscher et al. (2000) showed that sulfate reduction is directly correlated with calcium carbonate precipitation and the formation of micritic crusts in stromatolites.
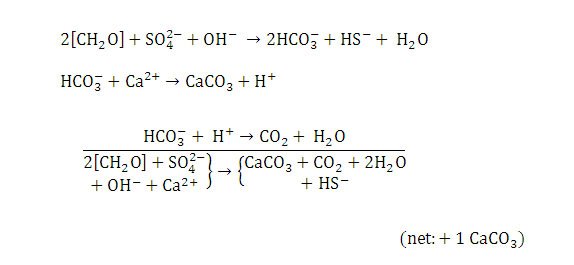
Figure 13. Chemical reactions resulting in calcium carbonate precipitation in Type 2 mats. Reprinted from Reaction 3, Visscher et al., 1998.
Lithification could be viewed as disadvantageous to microorganisms as they essentially become entombed in rock. However, there are advantages. In nutrient poor environments such as the open marine environment of the Bahamian stromatolites, this entombment essentially seals in nutrients and protects microorganisms from eukaryotic predators (Visscher & Stolz 2005). Another advantage comes from sulfate reduction and other reactions that result in calcium carbonate precipitation. These reactions result in the release of protons (H+) that form a proton gradient across the bacterial cell membrane (see previous equations). This generates a proton motive force that can be used by the bacteria for energy generation and other cellular processes (McConnaughey & Whelan 1997). The community of microorganisms working together in their respective “guilds” builds and lithifies the stromatolite for the purposes of protection, nutrition, and energy formation allowing microorganisms to survive in very harsh environments.
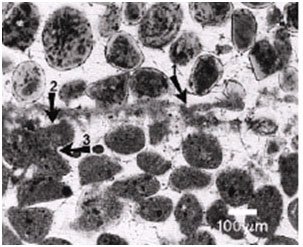
Figure 14. Light micrograph of Bahamian stromatolite. Arrow 1 points to the micritic crust. Arrow 2 points to a truncated micritized sand grain due to microboring. Arrow 3 points to fused or welded micritized sand grains that are believed to stabilize the stromatolite. Reprinted from Figure 3, Macintyre et al., 2000.
Sulfate reduction is the dominant metabolic activity by heterotrophic bacteria in Type 3 mats (Reid et al. 2000) (Figure 13). This activity is closely associated with the boring activity of the endolithic cyanobacteria Solentia sp. Solentia bore through sand grains that have been deposited during periods of sediment accretion (previous surface Type 1 mats) (Figures 14 and 15). The endolithic cyanobacteria leave behind abundant amounts of EPS that are then metabolized by heterotrophic bacteria, mainly SRB, in the boreholes (Reid et al. 2000). This results in calcium carbonate precipitation and the formation of micritized sand grains (Macintyre et al. 2000). The calcium carbonate is typically in the form of aragonite needles that are clearly visible in the infilled boreholes (Macintyre et al. 2000 and Reid et al. 2000). The metabolic activity of the heterotrophs and subsequent precipitation is progressive as the cyanobacteria bore through the sand grains (Macintyre et al. 2000).
The micritized sand grains become welded together as Solentia crosses between grains (Macintyre et al. 2000) (Figures 14 and 15). Boring, fusing and infilling are also observed in Hamelin Pool stromatolites (Goh et al. 2009; Jahnert & Collins 2012). Rather than boring being a destructive process it is actually a constructive, stabilizing, and preserving process due to the infilling and welding that accompanies the boring (Macintyre et al. 2000).
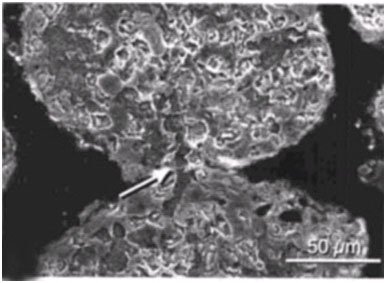
Figure 15. Scanning electron micrograph of Hamelin Pool stromatolite showing welding of micritized sand grains. Arrow points to infilled borehole that crosses the fusion point between the grains. Reprinted from Plate 48, Figure 1c, Reid et al., 2003.
Role of Environmental Factors in the Formation of Stromatolites
Metabolic activities of microorganisms are important factors in determining PML but environmental factors also play a role. A combination of both intrinsic and extrinsic factors leads to precipitation and mineralization resulting in lithification of the stromatolite.
Although varied terminology is used to categorize factors leading to mineralization in stromatolites, we will use that of Dupraz et al. (2009) as it is the most comprehensive. Dupraz et al. (2009, p. 144) uses the term organomineralization sensu lato to refer “to the process of mineral precipitation on an organic matrix, which is not genetically organized.” Organomineralization s.l. can be divided into two subcategories—biologically induced mineralization and biologically influenced mineralization (Dupraz et al. 2009) (Figure 16). Wright and Oren (2005) using the terms active precipitation (biologically induced) and passive precipitation (biologically influenced) suggest a similar division of mineralization processes.
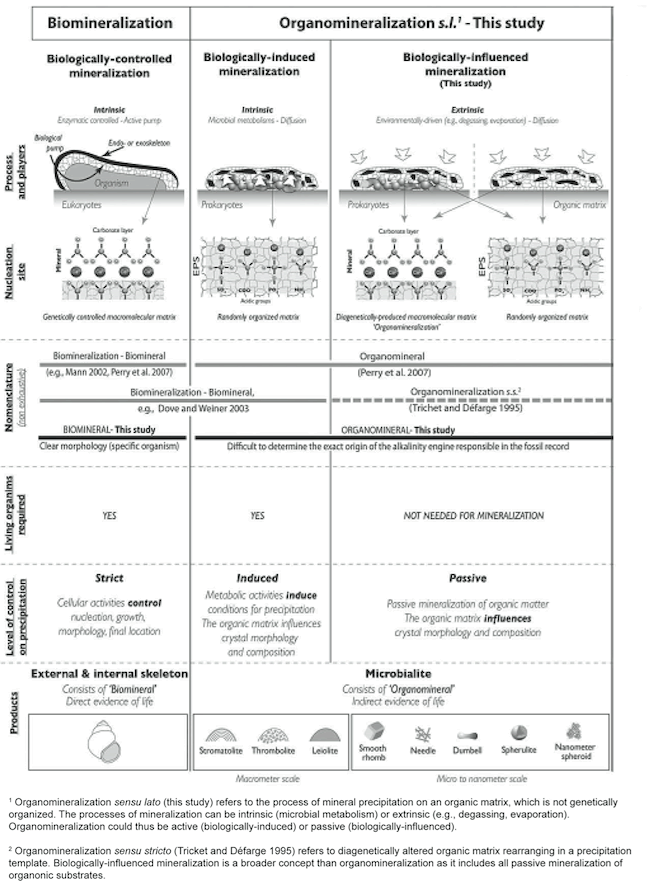
Figure 16. Classification of different types of biologically relevant mineralization. Organomineralization sensu stricto is represented in the first column on the left (not discussed in this paper) and organomineralization sensu lato is represented in the middle column and column on the right. Reprinted and adapted from Figure 2, Dupraz et al., 2009.
Biologically induced mineralization is the direct result of microbial metabolism changing the forms and balance of organic carbon (i.e. CO2 and carbohydrates) leading to conditions that result in calcium carbonate precipitation (Dupraz et al. 2009) (Figure 16), and was discussed in the previous section. Biologically influenced mineralization consists of environmental factors that are extrinsic to the microorganisms (Dupraz et al. 2009) (Figure 17). The so-called “alkalinity engine” determines carbonate alkalinity and is a major factor determining calcium carbonate precipitation. It is influenced by both intrinsic and extrinsic factors (Dupraz et al. 2009) (Figure 17). Intrinsic factors come from the microorganisms themselves (see previous section). Two major extrinsic factors are evaporation of water leading to the formation of evaporites and CO2 degassing (Dupraz et al. 2009) (Figure 17). Evaporites are salt deposits that can be composed of carbonate precipitates (Dupraz et al. 2009).
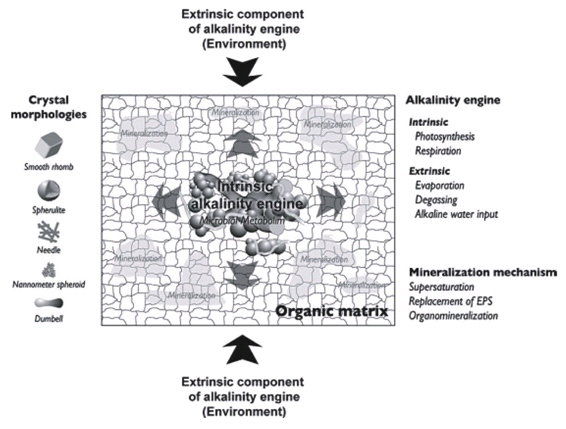
Figure 17. Effect of intrinsic and extrinsic factors on the alkalinity engine resulting in calcium carbonate precipitation and mineralization of the organic matrix mainly composed of the extracellular polymeric substance (EPS). Reprinted from Figure 4, Dupraz et al., 2009.
CO2 degassing (removal) causes a shift that favors calcium carbonate precipitation (Dupraz et al. 2009).
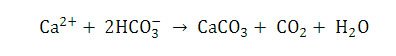
Both biologically induced and biologically influenced processes work together to create microenvironments that favor precipitation of calcium carbonate. Interestingly, normal seawater is supersaturated in relation to calcium carbonate (CaCO3) and dolomite (Ca,Mg(CO3)2) and therefore should spontaneously precipitate out of solution (Wright & Oren 2005). This is commonly referred to as the “dolomite problem.” Wright and Oren (2005) point out that certain kinetic barriers (i.e. the high enthalpy of hydration of the Mg2+ and Ca2+ ions) are in place to prevent the spontaneous precipitation of calcium carbonate. In the stromatolite it is believed that SRB remove these kinetic barriers and saturate the area around the cells with respect to carbonate (Wright & Oren 2005). This coupled with the release of calcium from the degradation of EPS (again mainly by SRB) and the “alkalinity engine” increasing calcium carbonate alkalinity results in a microenvironment that is favorable to calcium carbonate and dolomite precipitation.
Another aspect of the stromatolite microenvironment that mediates precipitation and mineralization is the organic matrix consisting mainly of the EPS (secreted by cyanobacteria). The EPS serves as a template or scaffold on which precipitation nucleates (begins) and grows (Dupraz et al. 2009) (Figure 18). The EPS matrix is replaced with small carbonate nanospherulites that are the result of precipitation and serve as a nucleation point for further crystal growth (Dupraz et al. 2009).
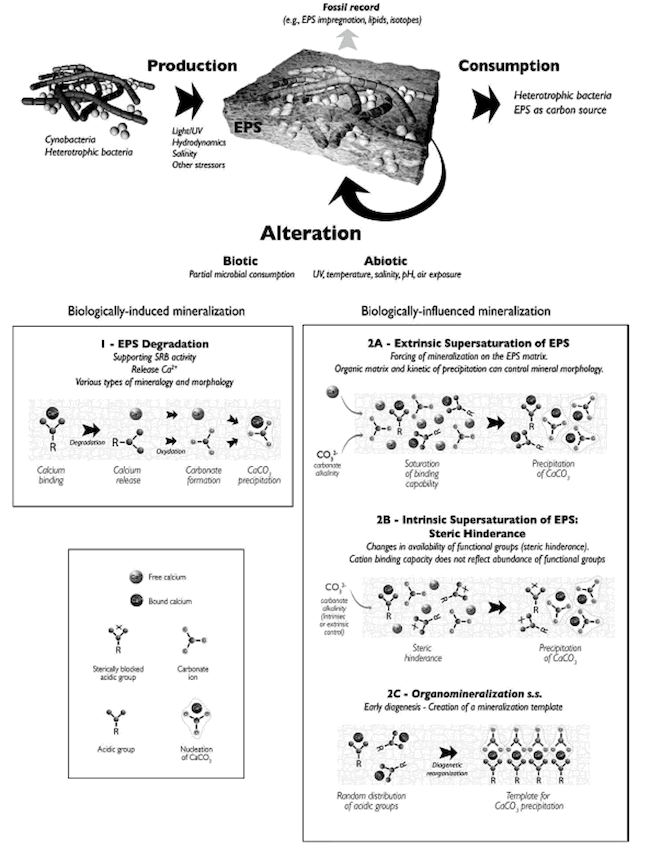
Figure 18. Effect of biologically induced and biologically influenced mineralization on the organomineralization of the EPS. Reprinted from Figure 6, Dupraz et al., 2009.
The organomineralization of the EPS is affected by biologically induced and biologically influenced factors (Dupraz et al. 2009) (Figure 18). As discussed previously, the EPS is degraded by SRB freeing calcium. In addition, the area in and around the EPS is supersaturated with respect to calcium thus favoring calcium carbonate precipitation (Dupraz et al. 2009) (Figure 18).
Distinctive calcium carbonate mineralogies associated with organomineralization (and typically not inorganic processes) are aragonite, calcite, monohydrocalcite, vaterite, and high Mg-calcite to Ca-dolomite (Dupraz et al. 2009). Kawaguchi and Decho (2002) found abundant aragonite needles embedded in the EPS matrix. Distinctive crystal morphologies associated with organomineralization (and not inorganic processes) are smooth rhombs, needles, dumbbells, spherulites, and nanometer spheroids (Dupraz et al. 2009) (Figure 16). These mineralogies and crystal morphologies are abundant in stromatolites indicating again the necessity of microorganisms and biological activity for the formation and lithification of stromatolites.
Understanding the processes of precipitation and mineralization resulting in lithification in living stromatolites is essential to develop criteria to determine the biogenicity of fossil stromatolites. For example, precipitation and mineralization occur as a result of degradation and modification of the amorphous EPS. Since precipitation and mineralization are not directly associated with bacterial structures (such as cyanobacterial sheaths) it may greatly diminish the number of microfossils associated with fossil stromatolites. Therefore, the absence of microfossils in fossil stromatolites is not necessarily an indicator that abiogenic processes formed them.
Developing Criteria to Determine the Biogenecity of Fossil Stromatolites
Given the general absence of microbial fossils within most fossil stromatolitic structures, it clearly is difficult, and perhaps impossible, to prove beyond question that the vast majority of reported fossil stromatolites, even those of the Proterozoic, are assuredly biogenic. Yet the Proterozoic stromatolites are so widespread and abundant (800 taxa in more than 600 stromatolitic rock units are known worldwide [Altermann 2004]), and their biological interpretation now seems to be firmly backed by studies of microbial communities cellularly preserved in Proterozoic cherty stromatolites (e.g., Mendelson & Schopf 1992; Schopf et al. 2005), so that many stromatolite workers believe that most are products of biological activity.
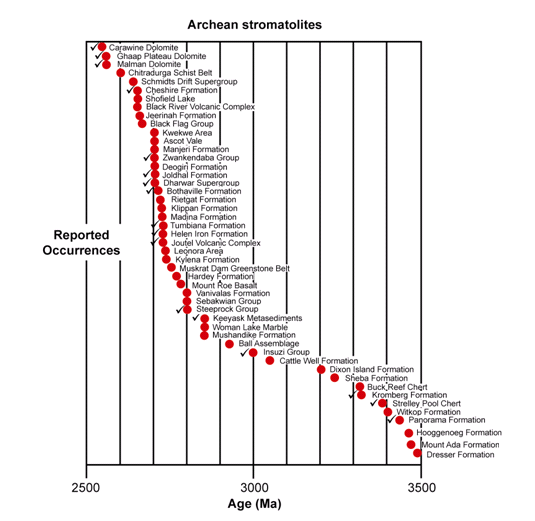
Figure 19. Stromatolite-containing Archean geologic units, the check marks denoting occurrences of conical stromatolites (after Schopf 2006).
In the Archean rock record, the problem of proving the biogenicity of such structures presents a greater challenge, due chiefly to the paucity of exposed Archean sedimentary strata (found only in the Pilbara Craton of Western Australia and the Barberton Greenstone Belt of South Africa and Swaziland) and the correspondingly small number of known occurrences of stromatolites (48 thus far) and preserved microbial assemblages (Figure 19) (Schopf et al. 2007). Nevertheless, Archean stromatolites are now established to have been more abundant and decidedly more diverse than was appreciated even a few years ago—40 morphotypes in fourteen Archean rock units (Hofmann 2000; Schopf 2006). Virtually all such structures that have been reported have also been studied in detail in Proterozoic stromatolites. Thus the interpretation of the biogenicity of Archean forms, and the differentiation of such structures from abiotic look-alikes, is based on the same criteria as those applied to stromatolites of documented biogenicity in the younger Precambrian (including analyses of their laminar microstructure, morphogenesis, mineralogy, diagenetic alteration, and more—e.g., Buick et al. 1981; Walter 1983; Hofmann 2000). All 48 occurrences of Archean stromatolites are regarded by those who reported them as meeting the biology-centered definition for stromatolites.
Others have also similarly studied living stromatolites in order to establish criteria for determining the biogenicity of fossil stromatolites. Their rationale, like ours, is that the characteristics observed in living stromatolites would be expected to be found in fossil stromatolites. In the geologic record there are of course non-stromatolitic rocks that contain the same bacterial body microfossils as found in some fossil stromatolites, so that begs the question as to whether the bacterial body microfossils always indicate a genetic connection between the bacteria that left the microfossils and the sedimentary and stromatolite structures. So there will always be a measure of subjectivity in using any list of criteria. Nevertheless, the final decision as to whether a fossil stromatolite is of biogenic origin will likely be determined on whether most of the identification criteria have been satisfied. Certainly, the presence of bacterial body microfossils in a fossil stromatolite has been regarded as logically desirable for it to be classed as of biogenic origin.
Known living stromatolites generally consist of carbonate sand-sized particles that have micritic laminae and crusts, whereas Precambrian fossil stromatolites generally consist of only very fine-grained micrite (calcium carbonate mud crystals <4 microns in diameter) (Riding 2000). While this difference could be used to question the biogenicity of the Precambrian stromatolites, it should be remembered that this difference is reflected in a comparable difference between the composition and constituents of modern and Precambrian sediments. Indeed, micritic textures are uncommon in most modern environments (not just stromatolite environments), whereas micritic textures are common in most fossil sediments (not just in stromatolites).
There have thus been numerous recent attempts to establish a set of criteria by which the biogenicity of fossil stromatolites may be determined, and these are now supported by appropriate diagnostic techniques (Grotzinger & Knoll 1999; Altermann 2004, 2008; Schopf 2004, 2006; Awramik & Grey 2005; Schopf et al. 2007; Noffke 2009). Our study of living stromatolites, and the work done by others to establish the biogenicity of various fossil stromatolites, has been used to assess and compile the following set of criteria. Among the crucial criteria for a fossil stromatolite to be of biogenic origin it must:
- Show a preferred orientation to the bedding of the sedimentary layer it is in;
- Show evidence of having been formed penecontemporaneously and synchronously with the sediment in the bed in which it is found, such as the layering within the fossil stromatolite consists of mineral grains that also constitute a major component of the sediments in the host bed;
- Be found in sedimentary rocks from the appropriate apparent depositional paleoenvironment, such as laminated limestones composed of lime silts, and cherts characteristic of peritidal and evaporitic carbonate environments;
- Be morphologically similar to living stromatolites in terms of the shape and geometry of its laminae having continuity across other structures;
- Have present within its laminae fossilized microbes with morphology (appropriate size and shape) consistent with microbes found in modern counterparts;
- Have associated microbial fossils that have the chemical composition of carbonaceous kerogen (and not graphite); and
- Have associated microbial fossils that have a carbon isotopic signature which matches the modern organisms with that morphology.
A systematic examination of fossil stromatolites applying these criteria to determine which are biogenic and which are not (Snelling, in prep.) is beyond the space and scope of this study. For our present purpose, having determined the suitable criteria to establish the biogenicity of fossil stromatolites, it will suffice to show that a sufficient number of Archean fossil stromatolites, including some of the oldest recognized occurrences, have been reasonably established as of biogenic origin. Those Archean fossil stromatolites of biogenic origin then are critical in understanding all fossil stromatolites of biogenic origin in the Creation-Flood framework of earth history (see below).
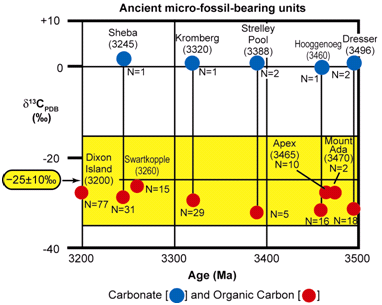
Figure 20. Carbon isotopic values of carbonate and organic carbon measured in bulk samples of the seven oldest microfossiliferous units known (after Strauss & Moore 1992; Schopf 2004).
The fossil stromatolites of the 3496 Ma Dresser Formation (in the Pilbara Craton of Western Australia) are the oldest known (Figure 19), and yet they have been established to be of biogenic origin due to their associated microfossils. Furthermore, these associated microbial fossils have the chemical composition of carbonaceous kerogen and have a carbon isotopic signature which matches similar modern microbes (Strauss & Moore 1992; Schopf 2004) (Figure 20), thus fulfilling criteria 5–7 above. Then in the case of the Archean 3430 Ma Strelley Pool chert (also in the Pilbara Craton of Western Australia) (Figure 19) a variety of fossil stromatolite morphotypes occur together in a pattern consistent with an interpreted stromatolitic reef ecosystem (Allwood et al. 2006, 2007). So coupled with the presence of associated fossilized microbes well-established by the chemical composition of the kerogen and their carbon isotopic signature (Figure 20), both of which are diagnostic (Schopf 2004, 2006; Schopf et al. 2007), the biogenicity of these Archean stromatolites would seem to be very firmly established.
Conical structures have been recorded in seventeen of the 48 units listed in Figure 19 (Hofmann 2000; Schopf 2006). Present in more than one-third of these deposits, notably including the 3430 Ma Strelley Pool chert (Hofmann et al. 1999; Allwood et al. 2006, 2007) and the Kromberg Formation (in the Barberton Greenstone Belt of South Africa and Swaziland) (Hofmann 2000), such coniform stromatolites appear to constitute a special case, distinctive structures evidently requiring for their formation both highly motile microbial mat builders and penecontemporaneous mineral precipitation (Grotzinger & Knoll 1999; Hofmann et al. 1999; Schopf 2006). Thus, Archean conical stromatolites, especially the conical structures found in the stromatolitic reef of the Strelley Pool chert, that can only have been produced by mat-building microbes because of there being no known sedimentological way of mimicking them, makes the biogenicity of these Archean fossilized stromatolites seem almost certain.
For some time stromatolite researchers thought that the microstructure of stromatolites was definitive for determining their biogenicity until more and more abiotic processes were found to create more and more stromatolite-looking textures (Lowe 1994; Grotzinger and Rothman 1996; Hladil 2005; Brasier et al. 2006). As a result, microstructures as a single crucial criterion for the biogenicity of stromatolites have become less and less persuasive, and so they should be considered non-definitive. However, as noted earlier, since precipitation and mineralization are not directly associated with bacterial structures (such as cyanobacterial sheaths) it may greatly diminish the number of microfossils associated with fossil stromatolites, and therefore the absence of microfossils in fossil stromatolites is not necessarily an indicator that abiogenic processes formed them. Thus the similarity in microstructures between modern stromatolites and Precambrian fossil stromatolites can still be a useful guide to gauge whether a fossil stromatolite warrants further investigation to find associated fossil microbes that might then help establish its biogenicity.
There is still the problem though of establishing a causal link between the microfossils found in fossil stromatolites and the building of the stromatolites themselves. Instead of the associated fossil microbes being the builders of the fossil stromatolites that enclose them, it could be argued that the original microbes were trapped in the stromatolite structures when they were being built by abiotic sedimentary processes. Nevertheless, the overall morphology (shape and size) of the stromatolites themselves, rather than just their internal microstructures (e.g. laminations), can still be a useful criterion for establishing the biogenicity of fossil stromatolites. For example, Wise and Snelling (2005) described a fossilized stromatolite reef in the Neoproterozoic Kwagunt Formation of the eastern Grand Canyon, consisting of in situ grown stromatolites side-by-side, and they concluded that these stromatolites were of biogenic origin. They did not see the need to demonstrate any causal link between the microfossils also found in the enclosing sediments and the stromatolites in this fossilized reef, because the morphology of the stromatolites and their relationship to one another in the reef were sufficient to establish the biogenicity of these stromatolites. Well-established microfossil and stromatolite associations are found throughout Proterozoic rock sequences, and again, while a causal relationship is difficult to establish, the biogenic origin of most Proterozoic stromatolites by microbial mat activity is not questioned, due to the morphology of the stromatolites in their sedimentary contexts being comparatively similar to modern living stromatolites, even though today’s microbial mat-builders are often not identical to the fossil microbes sometimes found associated with the fossil stromatolites.
Thus the listed criteria are not individually diagnostic of the biogenicity of fossil stromatolites. However, collectively they have enabled the likelihood of the biogenicity of many Precambrian stromatolites to be established. This process has been enhanced by the availability of newer technology to detect, identify and analyze the microbial fossils being found associated with an increasing number of fossil stromatolites (Schopf 2004; Schopf et al. 2005; Schopf 2006). Yet even though there are still many Precambrian fossil stromatolites whose biogenicity is thus far not firmly established, the fact that several of the oldest Archean fossil stromatolites have had their biogenicity well-established means that our efforts to understand and place Precambrian fossil stromatolites within the biblical framework of earth history is not dependent on establishing the biogenicity of every Precambrian fossil stromatolite.
Understanding Fossil Stromatolites in the Creation-Flood Framework
Since the biogenicity of many Archean stromatolites has now been well established in the relevant literature, it is important to grapple with how and where they fit within the biblical framework of earth history. Added to that is the compelling evidence of stromatolitic reefs that grew in place due to microbial activity as far back as 3430 Ma in the conventional geologic timescale. Wise and Snelling (2005) discussed the options for when Precambrian fossil stromatolites may have formed and under what conditions. There seems to be only three logical possibilities for their origin—they were created by God as fossils, or they were created by God as complete, fully functioning entities, or they developed as a result of natural post-creation, antediluvian growth processes. However, in the case of a given stromatolite all three could be true (a created fossil core, an initially created living stromatolite structure, and subsequent post-creation growth). Furthermore, the creation of a “fully functioning entity” would seem, almost by definition to have involved the creation of a fossil core and allowed for post-creation growth. These thus do not seem to be mutually exclusive possibilities.
Each of these options has its difficulties. Is it reasonable to assume that God would have created a whole Archean stromatolite reef in fossil form? If God created such fossil stromatolites already in fossil form, logically it could be postulated that God created all the fossils as they are. Where then in the geologic record would the fossils have changed from those directly created by God, to those produced by living creatures being buried and fossilized? Yet at some point this must seriously be considered as a possibility in the young-earth creationist model. The wine created by Jesus at the Cana wedding feast (John 2:1–10) simulated wine produced by secondary (natural) processes. Similarly, the bread and fish created by Jesus at the feeding of the 4,000 (Matthew 15:32–38) and the 5,000 (Matthew 14:15–21) also simulated bread and fish generated by secondary processes, and He didn’t deceive us about those events because they were recorded for us by eyewitnesses. Thus God does create objects which look like they developed by secondary processes.
The second logical possibility is that the stromatolites were created by God alive as fully functioning entities and then they were buried subsequently by ongoing sedimentary processes. However, given that above the earliest Archean (3.5 Ga) fossil stromatolites in the Western Australian Dresser Formation there is a very thick and extensive Precambrian rock sequence that also contains fossil stromatolites, including stromatolite reef structures spanning from other early Archean stromatolites through to those in the Neoproterozoic (Allwood et al. 2006, 2007; Wise & Snelling 2005), at what point in this strata record did God create the first fully functional living stromatolites? If He only created the earliest fossil stromatolites, those in the lower Archean rock units, then it might be argued that all those fossil stromatolites in the overlying rock units in these thick Precambrian rock sequences would have to have formed by “normal” secondary (natural) processes, which would seem to require an enormous timespan. Then again, there is the likelihood that some (or even many) of those overlying sediments were formed by reworking of previous sediments, and thus the stromatolites could similarly be reworked.
The third logical possibility is that God only created the mat-building microbes, and they then built the first stromatolites that were then buried and fossilized in the Dresser Formation. This possibility is only a small modification of the second possibility just discussed, with a small step back in time for God to create just the mat-building microbes rather than the complete stromatolites in a fully functioning reef. This possibility then requires an enormous timespan for all the subsequent stromatolites and stromatolite reefs fossilized in the thick overlying Precambrian rock sequences to grow and then be buried and fossilized, and/or be reworked.
The choice between these options therefore would seem somewhat arbitrary, since a case could be argued for each. But the difficulty of deciphering at what point in the fossil record is the boundary between the created fossils and then the fossils which formed from creatures that lived and were subsequently buried would seem to rule out the first option. Since the second and third options are closely similar, and God has shown us that He has created fully developed completed entities that appear to have been produced (according to human experience) by secondary processes (e.g. the wine at the Cana marriage feast), then it might be considered reasonable to start with the working hypothesis that God created the first stromatolites as fully functioning living entities, just as He created the first fish, birds, creeping things, beasts of the field, and Adam and Eve. This also makes good sense, in that when God made the soil on the land surface He developed on Day 3 of the Creation Week, He would have also created the microbes in the soil that are part of that fully functioning ecosystem (i.e. as part of the entity they are created in).
In the biblical geologic model of earth history proposed by Snelling (2009) it is postulated that the earliest rocks of the geologic record were created and put in place along with the earth’s fundamental internal structure during the first two days of the Creation Week. We are not told in the Genesis account what was under those globe-covering waters of those first two days, so we can propose the possibility of the first rocks making the earth’s earliest crust, which may have even included marine sediments covering a crystalline basement. Under a global ocean today one would expect marine sediments covering the ocean floor, and in shallower areas these sediments would be carbonates, even containing microbes. So if God created fully functioning entities during the Creation Week as described in the Genesis account, then it is reasonable to postulate that even before Day 3 He could have created the first fully functioning stromatolites with mat-building microbes, quite likely even including stromatolite reefs, with the sediments blanketing the global ocean floor. This is consistent with God creating soil microbes in the soils He made on Day 3.
Then on the Day 3 when God gathered the waters covering the earth into one place and formed the dry land, such actions required catastrophic earth movements to create and uplift a supercontinent. As the supercontinent breached the global ocean, the waters covering the emerging supercontinent were swept aside, and as they drained away they catastrophically eroded that emerging land surface. The sediments carried by those retreating waters in this “Great Regression” would have been deposited at the margins of the supercontinent, with diminishing quantities being spread thinly over the ocean floors around the rest of the globe. Since the emerging land surface would have originally been ocean floor covered in sediments from Days 1 and 2, including carbonates with stromatolites and stromatolite reefs, then these would also have been eroded and reworked. We may thus conjecture the possibility of continued catastrophic deposition from the retreating sediment-laden waters of the Great Regression beyond the continental margins through the remainder of the Creation Week and even into the early part of the pre-Flood era. Such catastrophic deposition would accomplish the rapid accumulation of the thick Archean to mid-Proterozoic sedimentary strata sequences, including entombed stromatolites and microbes, before quieter conditions prevailed offshore, where renewed growth of stromatolites and stromatolite reefs began again as mat-building microbes re-established themselves on and in the shallow offshore carbonate sediments.
This implies that the immense thick sequences of Precambrian sediments enclosing many repeated levels in which stromatolites are found fossilized over wide geographic areas can be fitted into the time period from Day 3 of the Creation Week through the 1650 or so years of the pre-Flood era. This requires mechanisms for both the accumulation of those thick Precambrian sedimentary strata sequences enclosing and fossilizing many stromatolites, microbes and stromatolite reefs reworked from those created prior to the Day 3 Great Regression growing in and on the carbonate sediments of the first global ocean floor, and then for subsequent penecontemporaneous and synchronous growth of stromatolites, microbes and stromatolite reefs in the quieter pre-Flood era offshore conditions, some also being buried and fossilized as pre-Flood sedimentation continued.
This proposed scenario then raises the question as to where in the geologic record the Creation Week-pre-Flood era boundary might be placed. The Scriptures refer to springs and fountains in the pre-Flood world (Genesis 2:6, 10–14; 7:11; Revelation 14:7), and the Archean-Proterozoic geologic record preserves evidence of the activities of those springs and fountains (Snelling 2009). If these springs and fountains were initiated by the catastrophic earth movements when God formed and uplifted the pre-Flood supercontinent on Day 3 of the Creation Week, and these waters were therefore initially very hot from the magmatic activity associated with those earth movements, then this would be reflected in the geologic record accumulated in this dynamic period through the latter part of the Creation Week. That the waters of these fountains and springs were hot and therefore mineral-laden is evidenced by the uniquely Proterozoic, massive banded iron formations (BIFs), volcanic and volcaniclastic strata, and the thick carbonate sedimentary units with their enclosed fossil stromatolites. Since these BIFs are unique to this section of the geologic record (Snelling 2009), it could be argued that they represent a unique period in earth’s history. And since their arguably rapid accumulation was accompanied by massive outpourings of volcanics and explosive volcaniclastics, it may be feasible to equate these and the BIFs as having accumulated rapidly during the dynamic period of the Day 3 Great Regression and its aftermath through the remainder of the Creation Week. This would then place the Creation Week-pre-Flood era boundary some place in the geologic record above these BIFs, perhaps even as high as the Paleoproterozoic-Mesoproterozoic boundary (1.6 Ga) (Gradstein et al. 2012).
Wise (2003) proposed that there were extensive fringing stromatolite reefs around the pre-Flood supercontinent. The reef-to-land “lagoon” was probably at least hundreds of kilometers wide, based upon the distribution of the extensive carbonate platforms on which carbonate sedimentary units accumulated during the Precambrian. These stromatolite reefs constituted a stromatolite-hydrothermal biome that only flourished in the pre-Flood era. The hot waters of the fountains and springs not only provided the nutrients for the rapid growth of the microbes responsible for building these fringing stromatolite reefs and the associated stromatolites that grew within the enclosed shallow lagoon waters, but also the voluminous dissolved minerals that were precipitated to accumulate the thick carbonate sedimentary units that entombed and fossilized the stromatolites. The Neoproterozoic Kwagunt Formation stromatolite reef described by Wise and Snelling (2005) would be the fossilized remains of an example of this pre-Flood stromatolite-hydrothermal biome.
The break-up of the pre-Flood supercontinent during the terminal Neoproterozoic at the initiation of the Flood would then have marked the almost complete demise of living stromatolites. The Flood cataclysm began with the breaking up of those fountains of the great deep, the collapse of the margins of the pre-Flood supercontinent, and the rifting of the supercontinent and the ocean basins. The Flood cataclysm then reshaped the earth’s surface as it deposited the Phanerozoic geologic record and entombed macrofossils in it. During the catastrophic conditions of the year-long Flood there were not sufficiently long timespans available for microbial activity on transient surfaces to develop into living and growing stromatolites of any significant thickness or geographic extent. This would explain the almost complete lack of fossil stromatolites of any significant thickness or geographic extent in the Paleozoic-Mesozoic strata record of the Flood catastrophe. The microbes which survived through the Flood then re-established their mat-building activities to again grow stromatolites during the waning stages of the Flood through to the present, as seen in the uppermost part of the Phanerozoic strata and fossil records. In the present world living stromatolite remnants are rare and geographically isolated.
Conclusion
Today’s rare stromatolites are being built by the metabolism of bacterial microbes in mat communities at the sediment surface-water interface in a few geographically isolated locations. Growth is by sediment grain trapping and binding principally by precipitation of calcium carbonate, resulting in a distinctive cyclical laminar microstructure as the microbial mats repeatedly re-establish themselves at the sediment-water interface. This laminar microstructure and the mat-building microbes are the distinctive features of living stromatolites that are essential criteria in determining whether the relatively abundant Archean-Proterozoic (conventionally 3500–541 Ma) fossil stromatolites are likewise of biogenic origin. A robust set of biogenicity criteria have been established, which include the identification of microbial fossils associated with fossil stromatolites that should be composed of kerogen and have an appropriate carbon isotope signature. Powerful diagnostic techniques that can establish the identity of genuine microfossils have thus confirmed the biogenicity of many genuine fossil stromatolites, and even stromatolitic reefs, as far back in the strata record as the lower Archean (conventionally dated as early as 3496 Ma).
Within the biblical framework of earth history microbes, fully functioning stromatolites and even stromatolite reefs may have been created by God before Day 3 of the Creation Week, as an integral component of the carbonate sediments on the floor of the global ocean. During the Day 3 Great Regression those carbonate sediments, stromatolites and stromatolite reefs could have then been eroded and reworked to be deposited in subsequent sedimentary rock layers, thus accounting for their occurrence in the thick Precambrian sequences. Once re-established in the pre-Flood era, mat-building microbes and stromatolites then flourished in reefs fringing the pre-Flood supercontinent and in the wide lagoons they enclosed, first as sediments were rapidly deposited off the pre-Flood supercontinent’s margins after the land emerged, and then continuing on through the pre-Flood era. Their rapid proliferation was facilitated by the mineral-laden hot waters from fountains and springs, which also precipitated the stromatolite-fossilizing extensive thick carbonate strata. The onset of the Flood cataclysm marked the demise of living stromatolites. Unable to establish themselves and grow during the devastation of the Flood, today’s rare stromatolites are still being built by those mat-building microbes that survived in a few isolated places with conditions suitable for their growth.
References
Allen, M. A., F. Goh, B. P. Burns, and B. A. Neilan. 2009. Bacterial, archaeal and eukaryotic diversity of smooth and pustular microbial mat communities in the hypersaline lagoon of Shark Bay. Geobiology 7:82–96.
Allwood, A. C., M. R. Walter, B. S. Kamber, C. P. Marshall, and I. W. Burch, 2006. Stromatolite reef from the early Archaean era of Australia. Nature 441:714–718.
Allwood, A. C., M. R. Walter, I. W. Burch, and B. S. Kamber. 2007. 3.43 billion-year-old stromatolite reef from the Pilbara Craton of Western Australia: Ecosystem-scale insights to early life on Earth. Precambrian Research 158:198—227.
Altermann, W. 2004. Precambrian stromatolites: Problems in definition, classification, morphology and stratigraphy. The Precambrian Earth: Tempos and Events, P.G. Eriksson, et al. Editors, pp. 516-539. New York: Elsevier.
Altermann, W. 2008. Accretion, trapping and binding of sediment in Archean stromatolites—Morphological expression of the antiquity of life. Space Science Reviews 135:55–79.
Andres, M. S. and R. P. Reid. 2006. Growth morphologies of modern marine stromatolites: A case study from Highborne Cay, Bahamas. Sedimentary Geology 185:319–328.
Awramik, S. M. and K. Grey. 2005. Stromatolites: Biogenecity, biosignatures, and bioconfusion. Proceedings of the SPIE, 5906:1–9.
Baumgartner, L. K., J. R. Spear, D. H. Buckley, N. R. Pace, R. P. Reid, C. Dupraz, et al. 2009. Microbial diversity in modern marine stromatolites, Highborne Cay, Bahamas. Environmental Microbiology 11(10):2710–2719.
Brasier, M., N. McLoughlin, O. Green, and D. Wacey. 2006 . A fresh look at the fossil evidence for early Archaean cellular life, Philosophical Transactions of the Royal Society B 361:887–902.
Buick, R., J. S. R. Dunlop, and D. I. Groves. 1981. Stromatolite recognition in ancient rocks: An appraisal of irregular laminated structures in an early Archean chert barite unit from North Pole, Western Australia, Alcheringa, 5:161–181.
Chivas, A. R., T. Torgensen, and H.A. Polach. 1990. Growth rates and Holocene development of stromatolites from Shark Bay, Western Australia. Australian Journal of Earth Sciences 37:113–121.
Dupraz, C., R. P. Reid, O. Braissant, A. W. Decho, R. S. Norman, and P. T. Visscher. 2009. Processes of carbonate precipitation in modern microbial mats. Earth-Science Reviews 96:141–162.
Dupraz, D. and P. T. Visscher. 2005. Microbial lithification in marine stromatolites and hypersaline mats. TRENDS in Microbiology 13(9):429–438.
Foster, J. S., S. J. Green, S. R. Ahrendt, S. Golubic, R. P. Reid, K. L. Hetherington, and L. Bebout, 2009. Molecular and morphological characterization of cyanobacterial diversity in the stromatolites of Highborne Cay, Bahamas. The International Society for Microbial Ecology Journal 3(5):1–15.
Goh, F., M. A. Allen, S. Leuko, T. Kawaguchi, A. W. Decho, B. P. Burns, et al. 2009. Determining the specific microbial populations and their spatial distribution within the stromatolite ecosystem of Shark Bay. The International Society for Microbial Ecology Journal 3:383–396.
Goh, F., S. Leuko, M. A. Allen, J. P. Bowman, M. Kamekura, B. A. Neilan, et al. 2006. Halococcus hamelinensis sp. Nov., a novel halophilic archaeon isolated from stromatolites in Shark Bay, Australia. International Journal of Systematic and Evolutionary Microbiology 56:1323–1329.
Gradstein, F. M., J. G. Ogg, M. D. Schmitz, and G. M. Ogg. 2012. The Geologic Time Scale Amsterdam: Elsevier.
Grotzinger, J. P. and A. H. Knoll. 1999. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annual Review of Earth and Planetary Sciences 27:313–358.
Grotzinger, J. P. and D. H. Rothman. 1996. An abiotic model for stromatolite morphogenesis. Nature 383:423–425.
Havemann, S. A. and J. S. Foster. 2008. Comparative characterization of the microbial diversities of an artificial microbialite model and a natural stromatolite. Applied and Environmental Microbiology 74(23):7410–7421.
Hladil, J. 2005. The formation of stromatactis-type fenestral structures during the sedimentation of experimental slurries—a possible clue to a 120-year-old puzzle about stromatactis. Bulletin of Geosciences 80(3):193–211.
Hofmann, H. J., K. Grey, A. H. Hickman, and R. I. Thorpe. 1999. Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geological Society of American Bulletin 111(8):1256–1262.
Hofmann, H. J. 2000. Archean stromatolites as microbial archives. Microbial Sediments, R. E. Riding and S. M. Awramik, Editors, pp. 315-327. Berlin: Springer-Verlag.
Jahnert, R. J. and L. B. Collins. 2012. Characteristics, distribution and morphogenesis of subtidal microbial systems in Shark Bay, Australia. Marine Geology 303–306:115–136.
Kawaguchi, T. and A. W. Decho. 2002. A laboratory investigation of cyanobacterial extracellular polymeric secretions (EPS) in influencing CaCO3 polymorphism. Journal of Crystal Growth 240:230–235.
Lowe, D. R. 1994. Abiological origin of described stromatolites older than 3.2 Ga. Geology 22:387–390.
Macintyre, I. G., L. Prufert-Bebout, and R. P. Reid. 2000. The role of endolithic cyanobacteria in the formation of lithified laminae in Bahamian stromatolites. Sedimentology 47:915–921.
Macintyre, I. G., R. P. Reid, and R. S. Steneck. 1996. Growth history of stromatolites in a Holocene fringing reef, Stocking Island, Bahamas. Journal of Sedimentary Research 66(1):231–242.
McConnaughey, T. A. and J. F. Whelan. 1997. Calcification generates protons for nutrient and bicarbonate uptake. Earth-Science Reviews 42:95–117.
Mendelson, C. V. and J. W. Schopf. 1992. Proterozoic and selected early Cambrian microfossils and microfossil-like objects. The Proterozoic Biosphere: A Multidisciplinary Study, J.W. Schopf & C. Klein, Editors, pp. 865-951. New York:Cambridge University Press.
Noffke, N. 2009. The criteria for biogeneicity of microbially induced sedimentary structures (MISS) in Archean and younger, sandy deposits. Earth-Science Reviews 96:173–180.
Papineau, D., J. J. Walker, S. J., Mojzsis, and N. R. Pace. 2005. Composition and structure of microbial communities from stromatolites of Hamelin Pool in Shark Bay, Western Australia. Applied and Environmental Microbiology 71(8):4822–4832.
Reid, R. P., N. P. James, I. G. Macintyre, C. P. Dupraz, and R. V. Burne, 2003. Shark Bay stromatolites: Microfabrics and reinterpretation of origins Facies 49:299–324.
Reid, R. P., I. G. Macintyre, K. M. Browne, R. S. Steneck, and T. Miller. 1995. Modern marine stromatolites in the Exuma Cays, Bahamas: Uncommonly common. Facies 22:1–18.
Reid, R. P., P. T. Visscher, A. W. Decho, J. F. Stolz, B. M. Bebout, C. Dupraz, et al. 2000. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 406:989–992.
Riding, R. 2000. Microbial carbonates: the geological record of calcified bacterial-algal mats and biofilms. Sedimentology 47 (Suppl. 1):179–214.
Schopf, J. W. 2004. Earth’s earliest biosphere: Status of the hunt. The Precambrian Earth: Tempos and Events, P. G. Eriksson, W. Altermann, D. W. Nelson, W. U. Mueller & O. Catuneanu, Editors, pp. 516–539. New York: Elsevier.
Schopf, J. W. 2006. Fossil evidence of Archean life. Royal Society of Philosophy Transactions Series B 361:869–885.
Schopf, J. W., A. B. Kudryavtsev, D. J. Agresti, A. D. Czaja, and T. J. Wdowiak. 2005. Raman imagery: A new approach to assess the geochemical maturity and biogenicity of permineralized Precambrian fossils. Astrobiology 5:333–371.
Schopf, J. W., A. B. Kudryavtsev, A. D. Czaga, and A. D. Tripathi. 2007. Evidence of Archean life: Stromatolites and microfossils. Precambrian Research 158:141–155.
Semikhatov, M. A. and M. E. Raaben. 1996. Dynamics of the global diversity of Proterozoic stromatolites, Article II: Africa, Australia, North America and general synthesis. Stratigraphic and Geological Correlation 4:24–50.
Snelling, A. A. 2009. Earth’s Catastrophic Past: Geology, Creation and the Flood, pp. 335–364, 613–793. Dallas, Texas: Institute for Creation Research.
Snelling, A. A. (in prep.). Fossil stromatolites and microfossils: The earliest in the geologic record and their implications for the biblical framework of earth history.
Stolz, J. F., R. P. Reid, P. T. Visscher, A. W. Decho, R. S. Norman, R. J. Aspden, et al. 2009. The microbial communities of the modern marine stromatolites at Highborne Cay, Bahamas. Atoll Research Bulletin 567.
Strauss, H., and T. B. Moore. 1992. Abundances and isotopic compositions of carbon and sulfur species in whole rock and kerogen samples. The Proterozoic Biosphere: A Multidisciplinary Study, J.W. Schopf & C. Klein Editors, pp. 709–798. Cambridge, Massachusetts: Cambridge University Press.
Trichet, J. and C. Défarge. 1995. Non-biologically supported organomineralization. Bulletinde l'Institut Océanographique (Monaco), Numéro Spécial 14:203–236.
Visscher, P. T., R. P. Reid and B. M. Bebout. 2000. Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 28:919–922.
Visscher, P. T., R. P. Reid, B. M. Bebout, S. E. Hoeft, I. G. Macintyre, and J. A Thompson, Jr. 1998. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas): The role of sulfur cycling. American Mineralogist 83:1482–1493.
Visscher, P. T. and J. F. Stolz. 2005. Microbial mats as bioreactors: Populations, processes, and products. Palaeogeography, Palaeoclimatology, Palaeoecology 219:87–100.
Walter, M. R. 1983. Archean stromatolites: Evidence of the earth’s earliest benthos. Earth’s Earliest Biosphere: Its Origin and Evolution, J.W. Schopf, Editor, pp. 187–213. Princeton, New Jersey:Princeton University Press.
Wise, K. P. 2003. The hydrothermal biome: A pre-Flood environment, Proceedings of the Fifth International Conference on Creationism, R. L. Ivey, Jr. Editor, pp. 359-370. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Wise, K. P. and A. A. Snelling. 2005. A note on the pre-Flood/Flood boundary in the Grand Canyon. Origins 58:7–29.
Wright, D. T. and A. Oren. 2005. Nonphotosynthetic bacteria and the formation of carbonates and evaporates through time. Geomicrobiology Journal 22:27–53.

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis

