Chapter 9
The Origin of Disease: A Creation Perspective, Part 2
Microbes Decay
Many people ask, “Why is there such suffering and death? Why were there plagues and pestilence throughout history, and why do they still exist in modern times?” It was not until after sin entered the world that some microorganisms became pathogenic, or harmful, to man. One explanation is that the good bacteria began to deteriorate or degenerate into the pathogenic bacteria. Genomic decay is one of the most likely theories for explaining the origin of bacterial pathogenicity. It is consistent with the creation idea of a degenerating creation; but the minor changes in the genetic code could have changed the course of human history. When a bacterium degenerates, it loses valuable information and must find other sources to survive. Bacteria generally are made as one cell; and, as they lose information from their genetic hardware, they consequently can no longer produce their own needed materials to synthesize cell parts. Since they lack information they have to gain that same information or the same materials that come from the information from some other creature (an animal or a person), and in doing so they cause disease symptoms.
What about evolutionary arguments? Since bacteria can add information, could this process be used as evidence for evolution? The answer is no. Evolutionists may argue that this is acquiring information, but the acquisition of small DNA segments is still leading to disease. A parallel process, for example, is how rust forms: rust is the addition of oxygen plus iron. This added information is not progressive evolution, but rather it is a deteriorating process (i.e., corrosion). The organization is going downhill!
The Genesis of Germs: From Mutualism to Parasitism
There is a fine line between wellness or health and disease. A distinction should be made between the infection itself, that is, the entrance or colonization of the microbe and the primary injury to the body tissues, and the clinical disease that it may bring. Over the millennia, the ability of man’s body to keep bacteria in check via anatomical boundaries and immune response, in general, has diminished. Today, we still see varying degrees of colonization of microbes, infection, and disease among people. The infection may occur for hours, days, months, or even years before disease symptoms are felt, and some symptoms are not noticed at all. The word infection is derived from Latin origins meaning “to mix with or corrupt.” Infection refers to the relationship between two organisms, the host and the microbe, and the competition for supremacy that takes place between them. A host whose resistance is strong remains healthy, and the microbe is either removed by the host or assumes a benign relationship with the host. By contrast, if the host loses the competition, then disease develops. The term disease (dis [against] ease) is from Latin that means “living apart,” a reference to the separation of ill individuals from the general population. Disease may be viewed as any change from the general state of good health. It is important to note that the words disease and infection are not synonymous; a person may be infected without becoming diseased.
Beneficial colonization and infection occurs today. Perhaps when looking at the numbers of these events and microbes involved, beneficial colonization can be seen as a much more frequent event than pathogenesis. The concept of infection in the host-microbe relationship is expressed in the body’s normal flora. The normal flora is a population of microorganisms that infect the body without causing disease. The relationship between the body and its normal flora is an example of symbiosis. In some cases the symbiosis is beneficial to both the body and the microorganisms; this relationship is called mutualism. For example, species of Lactobacillus live in the human vagina and derive nutrients from the environment while producing acid to prevent the overgrowth of other organisms. Escherichia coli is generally presumed to live in a mutualistic manner in the intestine of the body, because it pays us “rent” with the vitamins that are derived from the bacteria.
Pathogenicity refers to the ability of a parasite to gain entry to the host’s tissues and bring about a physiological or anatomical change resulting in a change of health and thus disease. The word pathogenicity is derived from the Greek term pathos, meaning suffering. The term pathogen has the same root and refers to an organism having pathogenicity. The symbiotic relationship between host and parasite is called parasitism. The word virulence is derived from the Latin term, virulentus, meaning full of poison, and it is often used to express the degree of pathogenicity. It is a matter of degree. An organism such as the Yersinia pestis, the agent of Black Death (bubonic plague) is said to be hypervirulent. Another highly virulent bacterium is the typhoid bacillus.
By comparison, agents such as rhinovirus that causes common colds or Streptococcus mutans that causes dental caries have low virulence. However, it should be noted that a genetic change could make an organism like E. coli turn from a beneficial bacterium to a highly virulent pathogen, E. coli 0157H7.
The Origin of Hansen’s Disease (Leprosy)
Leprosy has terrified humanity for thousands of years, and was reported as early as 600 B.C. in India, China, and Egypt. Hansen’s disease is still a major health problem in many parts of Africa, Asia, and Latin America. For many centuries, leprosy was considered a curse of God, often associated with sin. It did not kill, but neither did it seem to end. Instead, it lingered for years, causing the tissues to degenerate and deforming the body. In biblical times, victims were required to call out “Unclean! Unclean!” and usually they were also ostracized from the community. Many have thought this to be a disease of the skin. It is better classified, however, as a disease of the nervous system because the leprosy bacterium attacks the nerves. The agent of leprosy is Mycobacterium leprae, an acid-fast rod related to the tuberculosis bacterium. The Norwegian physician Dr. Hansen first observed this organism in 1873, and thus it was referred to as Hansen’s bacillus. Hence, leprosy is now referred to as Hansen’s disease. Leprosy is spread by multiple skin contacts, as well as by droplets from the upper respiratory tracts.
The disease has an unusually long incubation period of three to six years, a factor that makes its identification very difficult. The bacteria are also heat sensitive; therefore, the bacillus lives in the cooler regions of the body. Its symptoms start in the skin and peripheral nervous system, then spread to various cooler parts like the hands, feet, face, and earlobes. Severe cases also involve the eyes and the respiratory tract. Patients with leprosy experience disfigurement of the skin and bones, twisting of the limbs, and curling of the fingers to form the characteristic claw hand. Facial features accompany thickening of the outer ear and collapse of the nose.
It was Dr. Paul Brand’s (the late world-renowned orthopedic surgeon and leprosy physician) work with leprosy patients that illustrated, in part, why God permitted there to be pain in this world. The leprosy bacillus destroys nerve endings that carry pain signals; there is a total loss of physical pain in advanced leprosy patients. When these people cannot sense touch or pain, they tend to injure themselves. Tumor-like growths called lepromas may form on the skin and in the respiratory tract, and the optic nerve may deteriorate. The largest number of deformities develops from loss of pain sensation due to extensive nerve damage. For instance, inattentive patients can pick up a cup of boiling water without flinching. Many patients accidentally let hot objects burn their fingers. In fact, some leprosy patients have had their fingers eaten by rats in their sleep because they were totally unaware of it happening; the lack of pain receptors cannot warn them of the danger.
According to Dr. Brand, the best example in the Bible of a person with Hansen’s disease is the man with the withered hand (Mark 3:5; Matt. 12:13; Luke 6:10). The man with the “withered hand” most likely suffered from tuberculoid leprosy. The deterioration and shriveling of limbs is indirectly due to leprosy bacillus. Hansen’s disease causes poor circulation and the loss of control that provides life-sustaining materials (oxygen and nutrients) from the blood. Without blood there will be deterioration and a lack of harmony, order, symmetry, and design. The people suffering from Hansen’s disease injure themselves due to a lack of pain. The withered hand is due in part to the leprosy bacillus attacking the peripheral nervous system. This indirectly cuts the flow of blood to the hand; this results in atrophy. No touch and no pain in the skin lead to a lack of proper muscle use: misuse of limbs, injuries, and muscle paralysis.
Disease Focus 9.2
Diseases in the Bible
Two diseases found in the Bible, leprosy (Hansen’s disease) and plague, are discussed in this chapter because of their frequency and the genomic decay that has taken place in both germ agents. Plague and leprosy are used more times than any other disease in the Bible. Their use in modern times is different from biblical times, yet there is no doubt that they overlap and still illustrate important points today. The term plague (plague, plagued, plagues) (Hebrew = deber, maggephah, makkah, nega, and negeph; Greek = mastix, plege) occurs 128 times in the KJV Bible. However, these numerous mentions of the word actually include many different infectious diseases of today, such as bubonic plague. A plague may be any form of trouble or harassment, but the term most often has a reference to a disease of epidemic proportions. Also, it is used to refer to a disease’s occurrence and especially when it is fatal in its effects. The Book of Revelation speaks of many plagues in the last days.
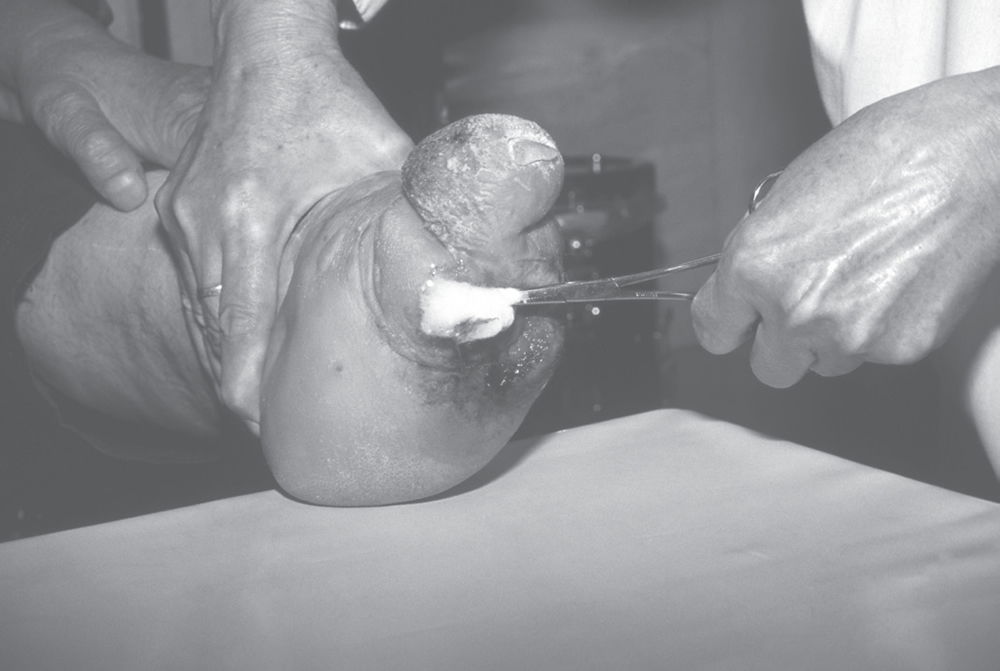
Leprosy. Cleaning the deformed foot of a person suffering from leprosy (Hansen’s disease) with an antiseptic. Leprosy is a chronic inflammatory disease caused by the bacterium Mycobacterium leprae.
The term leprosy (including leper, lepers, leprosy, leprous) occurs 68 times in the Bible: 55 times in the Old Testament (Hebrew = tsara’ath) and 13 times in the New Testament. In the Old Testament, the instances of leprosy that were mentioned most likely meant a variety of infectious skin diseases (including Hansen’s disease) and even mold and mildew when mentioned on clothing and walls. In the New Testament (Greek = lepros, lepra), it is then likely to be discussing Hansen’s disease. The precise meaning of the leprosy in both OT and NT is still in dispute. It is a comprehensive term that probably includes the modern Hansen’s disease (especially in the NT) and infectious skin diseases. The term Hansen’s disease was not given until 1873, when A. Hansen described the leprosy bacillus. Only at this point was a precise definition for leprosy made available. Word meanings do change; for instance in the days of Pasteur, the terms virus, bacterium, and fungus were used interchangeably. Meanings today are used because specific causes for infectious diseases were determined.
Mycobacterium leprae: A Decaying Creation
The best example of a bacterium undergoing genomic decay is Mycobacterium leprae (Disease Focus 9.3). The leprosy bacillus has a long incubation time and a total dependence upon living in animal or human tissue. The sequencing of M. leprae bacillus has given us insight into why this is the case. The sequencing of M. leprae has revealed that approximately 25 percent of functional genetic information has been lost. The extreme genomic decay that it has undergone has resulted in extensive metabolic constraints and an unusually slow growth rate. God has given people the understanding to discover many of the secrets of the mycobacterial genome that may be used to restore health.
This acid-fast bacillus has an incredibly slow doubling time of about 12 days and cannot be cultured in any standard medium; it can only be cultured in armadillos and the hind footpads of mice. Since 30°C is the optimum growth temperature for M. leprae (most of the human body is 37°C), it grows only in cooler regions of the body, such as the peripheral nerves, skin, testes, and mucous membranes of the upper respiratory tract, primarily the nose. It is transmitted via aerosol, droplets, and prolonged direct contact. It resides within the macrophages and Schwann-cells, and cannot survive in the extra-cellular environment. The destruction of the Schwann-cells is what causes the nerve damage resulting in an accompanying loss of sensation that is characteristic of Hansen’s disease.
Disease Focus 9.3
Mycobacterium leprae: The Details of Decay
Comparative genomics have contributed to a greater understanding of the leprosy bacillus. Major breakthroughs occurred by comparing the genomes of Mycobacterium leprae and its closest relative, M. tuberculosis. Gene clones from M. leprae in M. smegmatis (i.e., a nonpathogenic Mycobacteria) were used to determine the functionality of similar genes. The evaluation of the genomes of M. leprae, M. smegmatis, and Mycobacterium tuberculosis has revealed the necessary biosynthetic pathways. These comparisons have revealed that M. leprae has lost well over 2,000 genes (~25 percent of its total genome) since decaying from an original Mycobacterium species.
In order to determine the extent of decay of the leprosy bacillus, the genomes of M. leprae and M. tuberculosis chromosomes were used for comparison. Biologists believe that M. leprae has arisen from M. tuberculosis or a similar ancestor. M. leprae has undergone extensive devolution in metabolic and respiratory functions which then cause serious energy limitations. Compared to the 4.41-megabase (Mb) genome of M. tuberculosis, the 3.27 Mb genome of M. leprae is quite conserved. M. leprae possesses only 1,614 functional genes, compared to approximately 4,000 functional genes in M. tuberculosis. The M. leprae genes are denser than those of M. tuberculosis. M. tuberculosis has 4,411,532 base pairs (bp) compared to the 3,268,203 DNA base pairs in M. leprae. However, M. tuberculosis only contains 6 nonfunctional genes (i.e., pseudogenes), whereas the genome of M. leprae consists of 1,133 pseudogenes. M. tuberculosis has 4,025,965 protein coding bases; in contrast, M. leprae has 1,626,387 protein coding bases.
M. leprae contains 75 percent of the genes required for optimal growth in M. tuberculosis. There is only a 27 percent correspondence for genes non-essential to growth, thus M. leprae conserves a minimal amount of genes required to reproduce. This minimal genome is the cause for M. leprae’s inability to be grown in a Petri dish and characteristically slow growth in laboratory animals. Some of the major pathways lacking in M. leprae include synthesis of vitamin B-12 (i.e., cobalamin) and cell wall components (i.e., mycobactin and sulfur). Many genes that are central in metabolism are essential for growth. Some of the primary genes include those required for synthesis of amino acids, nucleic acids, and key cellular processes, including cell division, protein synthesis, replication, iron (i.e., siderophore) production, and transcription. Many of the genes required for growth in M. tuberculosis are constrained in M. leprae.
Cell Wall and Cell Processes Lost
In M. tuberculosis there are 751 genes devoted to the cell wall and cellular processes; however, M. leprae only posseses 371 genes for these functions. The cell wall (and its components) is essential for growth and survival. The inner layer of mycobacterial cell wall is composed of peptidoglycan, and the outer layer consists of mycolic acid. Mycolic acid is needed for a firm cell wall, and it performs other functions. The genes that typically code for this are mycolic acid that are both pseudogenes in the leprosy bacillus.
Two specific genes that are critical for coding the enzymes that are necessary for growth have been lost as well. Mycobacterium smegmatis (fast growing) and M. tuberculosis are found in M. bovis, M. avium, and M. leprae. The portion of the M. leprae genome that codes for proteins, sugars (polysaccharides), lipids (fat), peptidoglycan, and other chemicals consists of approximately 260 functional genes and approximately 76 pseudogenes. According to this data, the efficiency of the cell wall construction should be reduced by approximately 25 percent.
M. leprae contains about 110 functional genes devoted to cell functions, which would include cell division, adaptations to atypical conditions, detoxification, and production of amino acids, fatty acids, efflux proteins, and various ions. Approximately 25 percent of the genes responsible for cell division are non-functional. Over 50 percent of the genes accountable for detoxification are pseudogenes. About 50 percent of the genes that code for adaptations and atypical conditions, amino acids, proteins, and ions have decayed to a non-functional state. There are no functional genes for fatty acid transportation. Though M. leprae has decayed significantly, it still possesses enough information to produce its necessary components, such as lipids, amino acids, and DNA. It can also directly obtain these components from the host cell. It appears that the most significant result of the minimal genome is an incredibly slow growth rate.
Energy Metabolism
Intermediary metabolism and respiration is coded for by 895 genes in M. tuberculosis, but only 431 genes are present in M. leprae. There has been a reduction in the ability to break down sugars through glycolysis (anaerobic respiration) and other metabolic pathways. Two-fifths of the genes coding for the pentose phosphate pathway have diminished to pseudogenes. All of the genes that would be devoted to anaerobic respiration have corrupted into non-functional counterparts, therefore M. leprae possesses no anaerobic or microaerophilic capabilities. Furthermore, one-fourth of the bacillus’ genes devoted to aerobic respiration have also decayed to pseudogenes. With so many of the major metabolic functions being greatly reduced or even eliminated, M. leprae would have a slow growth rate. It takes 12 days for one bacterium to make another. (Recall, most bacteria take 20 minutes for binary fission, a type of asexual reproduction.) Also, the malic enzyme, which has been connected to fast growth in Mycobacteria, is lacking in M. leprae. It takes 3 months to 20 years (and the record is 40 years) for an infection to cause disease. However, once the leprosy bacillus is established in the body, it is difficult to eradicate. The growth is slow, but steady and sure.

Jesus Healing the Leper, 1864 (oil on canvas). Musee des Beaux-Arts, Nimes, France
Mycobacterium leprae has undergone extensive decay compared to other Mycobacteria species. M. leprae possesses the minimal essential genes necessary for the biological and structural properties in the Mycobacteria kind. It possesses the “essential’” gene set to survive, but little else. This is in contrast to Pseudomonas aeruginosa that has a large genome of over six MB, making it the largest known set of genes for a bacterium—and it grows on everything. The deterioration is likely due to the Edenic curse, which is consistent with a degenerating creation.
After 1981, the World Health Organization recommended multi-drug therapy (MDT) for those infected with M. leprae. This therapy consisted of the antibiotic trio of clofazimine, rifampicin, and dapsone. They were found very effective in curing Hansen’s disease in as few as six months. The world has seen dramatic reduction of the infection. Since 1985, the prevalence rate has dropped 90 percent, and 14 million people have been cured. Leprosy has been eradicated from 113 countries. Since 2001 there has been a 20 percent annual decrease in new cases detected. This cleansing of leprosy in the modern world may be a picture of how someday The Great Physician will restore our bodies in heaven. Just as Jesus restored the man with the withered hand, He will also restore His decaying creation to a good state once again (Luke 6:6).
Disease Focus 9.4
Biblical Leprosy and Hansen’s Disease
Biblical leprosy and Hansen’s disease have in common that both are dreaded and people were shunned because of them. The noun tsara’ath appears about two dozen times in the Hebrew Bible. It is most frequently seen in Leviticus, where it is used to describe a state of defilement manifested as a scaly condition of the skin, a condition of cloth, leather, and the walls of houses. In the Septuagint, the Greek translation of the Hebrew Bible, tsara’ath was translated as aphe lepras. These words in Greek implied a skin condition that spread over the body. Tsara’ath has continued to be translated as “leprosy,” even though this term is broader; as there was no leprosy as we know it in the Middle East during the time period the Hebrew Bible was written. Others have suggested that the translation of tsara’ath includes “molds.” The recent discovery of a highly toxic mold (Stachybotrys sp.) that contaminates buildings and causes respiratory distress, memory loss, and rash lends support to the translation of tsara’ath to include “mold.” Most likely tsara’ath incorporates a “collection” of contemporary terms, including Hansen’s disease, infectious skin diseases, and mold (or even mildew) diseases.
Biblical leprosy is a broader term than the leprosy (Hansen’s Disease) that we know today. The scholars who first translated the Bible from Hebrew to Greek used the term lepra when faced with the untranslatable Hebrew word tsara’ath. The writers were not medical students but good observers who recorded what they saw. The Hebrew tsara’ath included a variety of ailments. It referred primarily to uncleanness or imperfections according to biblical standards. A person with any skin blemish was tsara’ath. The symbolism extended to rot or blemish on leather, houses, and woven cloth. Other Old Testament references to leprosy are about punishment or consequences of sin. Balance these passages against others where God sent different afflictions for disobedience: the plagues on Pharaoh and Egypt, the Bubonic plague of the Philistines, the foot disease of King Asa of Judah. Leprosy is not singled out from other afflictions.
References to leprosy have a different emphasis between the Old and New Testaments. The Hebrew word tsara’ath and references to leprosy throughout the Old Testament have two particular contexts: 1) reference to ceremonial laws and ritual uncleanness and 2) punishment or for consequences of sin. All New Testament references to leprosy are in the Gospels and in the context of healing and social well-being. Jesus touched people with leprosy. People with leprosy traditionally have suffered banishment from family and neighbors. Jesus broke with the tradition; He treated people with leprosy by touching and cleaning them. Jesus had compassion on those who had leprosy.
Gene Swapping: Horizontal Gene Transfer Among Bacteria
Bacteria are quick-change artists. They have to be. Without warning, a person consumes a bacterium living in the dressing on a salad bar, and then it is transported to the stomach, which is highly acidic. Or a bacterium about to be injected into a human arm by an insect will soon encounter an environment very different from the one inside the insect. Only the adaptable survive. Bacteria change, not only by mutating their genomes but also by receiving and incorporating DNA segments from members of other species.
How can one define a single bacterium species if large portions of that bacterium’s genome are cobbled together in gene segments acquired from a mixture of other bacterial species that are not its direct ancestors but its contemporaries? Bacteria are using this capacity for acquiring genes from other organisms to allow them to adapt to rapidly changing environments and to become resistant to antibiotics. This rapid-change ability makes them capable of causing new, emerging, and re-emerging diseases. It facilitates and increases their options for survival.
Bacterial reproduction is usually described as asexual, because bacteria have no equivalent of the genetic fusion of two different cells that is characteristic of sexual reproduction in eukaryotes. Nonetheless, bacteria do have the ability to exchange segments of DNA with other bacteria. Because these segments can become fixed in a bacterium’s genome and confer new traits, gene exchange among bacteria could be considered to be a form of bacterial sex. DNA exchange between bacteria is called horizontal gene transfer to differentiate it from vertical gene transfer, the inheritance of a gene(s) from a progenitor. It is now known that many infectious diseases are due to the horizontal transfer of not just one gene, but many genes in clusters called cassettes, or pathogenicity islands.
The packages of genes frequently are transferred via plasmid DNA that code for enzymes, toxins, virulence factors, and proteins that lead to misery in the host recipient of germ-causing microbe. They are a corruption of the original good plan of the Creator. Corruption is the act of changing, or of being changed, for the worse; departure from what is good, or the ideal design by the Creator in Genesis 1 and 2. Additional information contaminates an otherwise good message.
For decades, bacteria have been known to be capable of horizontal gene transfer in the laboratory. In fact, information about horizontal gene transfer was the foundation of the genetic engineering revolution. Only when biotechnologists were able to insert foreign DNA into bacteria in the laboratory did cloning (via transformation) become possible. Until recently, however, many biologists have downplayed the importance of horizontal gene transfer as a form of bacterial variation in nature. However, new research on bacterial genomes and the discovery of what makes them pathogenic have provided evidence that the horizontal (not vertical) changes are what make bacteria different. In addition, the evidence is accumulating to support the contention that not only does horizontal gene transfer occur frequently in nature but that it plays a significant role in bacterial cell variation, corruption, and devolution (a form of variation).
The Origin of “Super Staph”—Methicillin Resistant Staphylococcus aureus (MRSA)
Staphylococcus aureus is a bacterium that can live harmlessly in the nose and occasionally on the skin in a state known as colonization. Most likely, S. aureus was originally designed to live in harmony with man as a harmless, normal flora. Perhaps it played a positive role in the recycling of elements in nose or skin as its “cousin,” S. epidermidis, did in its original good design. Today, S. aureus causes many diseases, including skin boils, infection of leg ulcers and pressure sores. Occasionally, it can cause more serious disorders such as blood poisoning (septicemia) and bone, joint, or heart valve infection. It is the number one hospital acquired (nosocomial) infection. Up until recently, commonly used antibiotics, such as penicillin, killed most strains of Staphylococcus aureus.
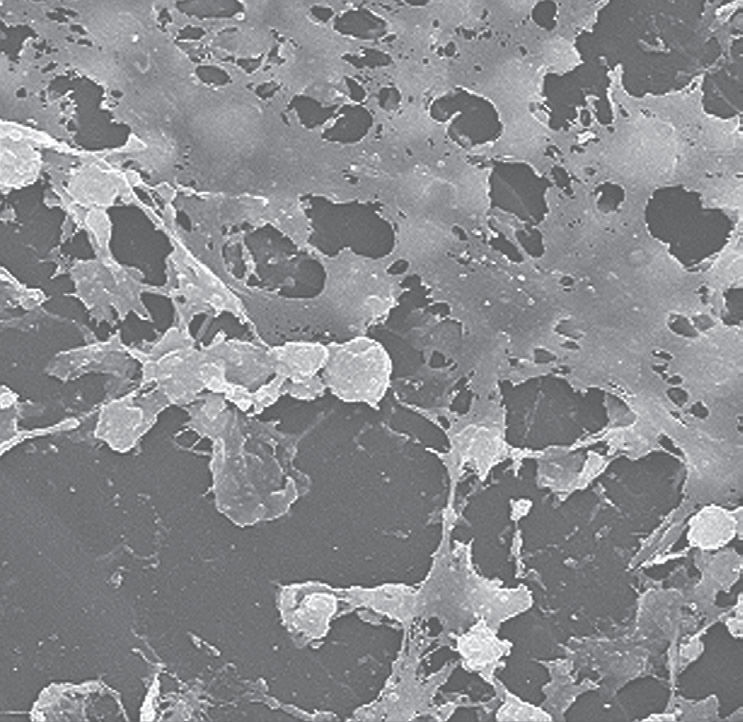
Scanning electron micrograph (SEM) of Staphylococcus aureus bacteria the sticky-looking substance woven between the round cocci bacteria, and is known as “biofilm.” This biofilm has been found to protect the bacteria that secrete the substance from attacks by antimicrobial agents such as antibiotics.
Now, S. aureus is resistant to many antibiotics, including methicillin. Although methicillin-resistant Staphylococcus aureus (MRSA) can also live harmlessly in the nose or skin, if infections develop they can be more difficult to treat. In addition, some types of MRSA appear to spread easily between patients in the hospital who then might become ill as a result. MRSA is sometimes called “Super Staph” or the “Superbug” in the press because of its resistance, its ability to cause infections in hospitalized patients, and its capacity to cause outbreaks on wards. The reason that hospitals seem to be hotbeds for resistant MRSA is because so many different strains are being thrown together with so many doses of antibiotics, vastly accelerating this natural selection process.
Resist Saying, “This Is Evolution”
Although MRSA’s antibiotic resistance and toxin genes appear to have been added to its existing plasmids, it has lost some key metabolic functions to survive on its own. S. aureus has picked both its resistance (R) genes and toxin genes from a pathogenicity island of Enterococcus faecalis (see Focus 9.4). This new information is a corruption of original plasmid DNA. This horizontal transfer does not improve the overall fitness of the bacteria. This change has allowed the bacterium to thrive primarily in hospital environments (number one nosocomial infection in some hospitals). However, it has reduced cellular functions in the wild, altered enzyme activities, and in some S. aureus mutations, eliminated expression of the normal genes.
While such changes in MRSA can be regarded as “beneficial” to bacterium because they increase the survival rate of bacteria in the presence of the antibiotic, they involve mutational processes that do not provide a genetic mechanism for real evolution (i.e., molecules to man). You should resist saying “evolution”; there is a loss of total function, and MRSA will lose its ability to compete with other bacteria in the wild and in a non-antibiotic environment.
The Origin of Bubonic Plague
Although some forms of the bacteria Yersinia are harmless, other forms have devastated human populations, with a plague of biblical proportions (Ps. 91:3–7, 9,10). Bubonic plague (also known as the “Black Death” that killed one fourth of Europe’s population in the 1300s) appeared as a great pestilence several times in the Old Testament, including in Psalm 91 and in 2 Samuel 24:14–25. Perhaps the clearest example of such a plague is recorded in 1 Samuel 6:4–19, where there is a specific reference to the tumors on people (bubos = the tumors of lymph glands) and to rats (the animal vector that carried the plague bacterium, Yersinia pestis). The biblical timeframe for the plagues described in 1 Samuel was about 3,000 years ago. Interestingly, experts on plague “evolution” estimate the emergence of Y. pestis about 1,500–20,000 years ago.
Plague’s Origin is Multifaceted
Many infectious diseases can be traced back to the decay and corruption of the original created design of microorganisms as a result of the Fall. Corruption literally means to destroy (from the Latin, corruptus). In biological terms, corruption is a loss of genetic information or an inappropriate addition of genetic material; whether an insertion or a deletion, the result is damage to the original code. The origin of pathogenic (disease causing) bacteria such as Y. pestis is both complex and multifaceted, and it may be explained by a combination of genes that were lost, added, and moved. The story of Yersinia’s degeneration into the plague pathogen may serve as a model of “fast” genomic decay and corruption.
It appears that the beginning of pathogenicity in the genus Yersina started with a net loss of chromosomal DNA from its original “kind.” Later in time, there were minor additions of plasmid DNA as well as viral and bacterial DNA. A few plasmid genes for toxins have been acquired from other existing species, but many chromosomal genes were lost. It takes only a few such gene changes to produce this new, extremely infectious variant, so it may have taken only hundreds or a few thousand years to produce the current bubonic plague strain that has existed for about the last 500 years.
Loss of Chromosomal DNA
Researchers hypothesize that key chromosomal genes (i.e., involved in metabolic pathways) were lost in the change from a soil inhabiting Yersinia type to a pathogenic Yersinia species. Pathogenic Yersiniae have lost the genetic expression of numerous (about 149) genes. Of the genes lost, 58 are the result of frameshift mutations, 32 have undergone deletions, and the rest are nonsense mutations—all of which prevented pre-existing genes from being normally expressed.
An important feature of the Y. pestis genome is the presence of pseudogenes. Biologists consider that these genes reflect a loss of structural information and function. Dr. Wren, the leading plague expert in the world, suggests that the genes lost in Y. pestis affected bioenergetic functions, including dicarboxylic amino-acid metabolism. This reduction of metabolic pathways may allow the bacterium to conserve energy. The newly emerged strains (variants) are streamlined and might contribute to the development of pathogenicity (i.e., plague) due to the genes they lack. The absence of important biosynthetic genes is believed to be a hallmark of genomic decay.
Genes Added and Moved
The corruption by three genes of a relatively benign recent ancestor of Y. pestis may have played a key role in the emergence of bubonic plague. A recent report by Hinnebusch and colleagues, a plague expert team at the National Institutes for Health, suggests that the acquisition of two plasmid genes (i.e., just a few discrete genetic changes) in recent times changed the fairly harmless Y. pseudotuberculosis, which caused mild food poisoning, to the agent of the “Black Death.” A third gene (carried on plasmid pMT1) produces murine toxin, an enzyme required for the initial survival of Y. pestis bacilli in the flea midgut (Table 9.1). By acquiring this gene from another organism, Y. pestis made a crucial shift in its host range. The bacterium now could survive in fleas, and it devolved to rely on its blood-feeding host for transmission. This is just another example of the flexibility of many microbes and how they sometimes repackage themselves into more dangerous agents of infectious disease.
Table 9.1. Virulence-associated plasmids of Yersinia pestis
| Plasmid Size | Transferred Factor | Action |
|---|---|---|
| pPla (9.6 kb) | Pla surface (protease) | activates plasminogen activator; destroys C3B; C5A (i.e., complement factors) |
| pYV (70.3 kb) | Yops (proteins) | interferes with phagocytosis and immune system |
| pMT1 (96.2 kb) | phospholipase D (Murine toxin) | bacterial transmission in fleas |
This last corruption is one that distinguishes Yersinia pestis from all closely related, more benign bacteria such as Y. pseudotuberculosis and other Yersinia (e.g., Y. entercolitica). In turn, as Y. pestis adapted to rely on its new blood-feeding host for transmission, the emergence of more deadly bacterial strains would have been favored. It appears that these minor plasmid additions were the last changes made in an otherwise long series of genetic losses in Y. pseudotuberculosis’ chromosome. One pathogenicity island was acquired by Yersinia pestis from another bacterium. This cassette of genes was not the evolution of new chromosomal DNA; it was an acquisition through lateral gene transfer. It produced a corrupted message that gave bacteria a new “position” in the gut. Y. pseudotuberculosis lacks a position gene (i.e., hms locus gene), which encourages the bacilli to remain harmlessly in the midgut of the flea. Plague bacilli, by contrast, have this inserted locus gene. Free from their original control and causing a lack of “good direction” information, the bacteria migrate from the midgut to the foregut and then form a plug of packed bacilli passed on to the victim.
Genes, Germs, and Genesis
Plague bacteria are not the only microorganisms that have degenerated into disease-causing organisms. Other pathogenic bacteria that have undergone genomic decay include various mycoplasmas (e.g., Mycoplasma genitalium and M. pneumonia, the latter causes pneumonia) and Mycobacterium leprae (the leprosy bacillus).
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- © 2024 Answers in Genesis

