
Like Dolly, Only Human
How They Cloned Humans, and Why They Should Stop
Abstract
Human clones may provide another way to make patient-matched embryonic stem cells . . . but at what price? Efforts have long been underway to produce human embryos through cloning. Some have hoped these embryonic clones would provide an ethically-neutral source of embryonic stem cells. Others hope these embryos can be brought to birth, providing reproductive technology without the necessity of fertilization by sperm. But many have expressed concern that such cloned people would be exploited. This concern for human clones should stretch back to humans at their earliest time of life—as embryos. An examination of the history and procedures involved in producing these clones reveals that human embryonic clones are just as human as embryos produced through in vitro fertilization. It is wrong to create humans in order to kill them.
Introduction
Has the day of The Island—the 2005 movie portraying the fate of personalized human clones made to supply replacement parts for affluent clients—nearly arrived? The human cloning method developed by Shoukhrat Mitalipov’s group at the Oregon Health & Science University isn’t ready to churn out walking, talking clones, but researchers believe it will ultimately supply made-to-order personalized human embryonic stem cells for research and medical treatment. The “recipe’s” success also re-ignites the bioethics of the human embryonic stem cell debate at a whole new level.
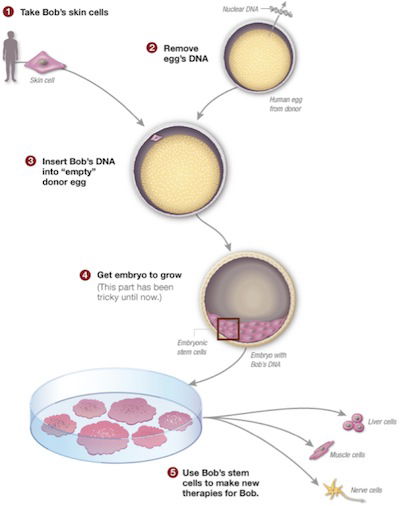
Researchers make clones of “Bob” by replacing the DNA of a donor egg with the DNA from one of Bob’s skin cells. Skin cells are a kind of somatic cell, so this is called somatic cell nuclear transfer (SCNT). The researchers stimulate the fused cell to become an embryo. Bob’s DNA blueprint directs this embryo to be human and genetically identical to Bob. The embryo’s “inner cell mass” contains embryonic stem cells (ESCs). These cells are harvested and the embryo is killed. The ESCs can then be grown and possibly used in an effort to develop medical treatments for Bob. Because the DNA in ESCs, like the DNA in induced pluripotent stem cells (iPSCs) matches Bob’s DNA, scientists hope Bob’s immune system won’t reject these cells. Image by Mitalipov Lab, Oregon Health & Science University, adapted for NPR by Alyson Hurt, via NPR.1
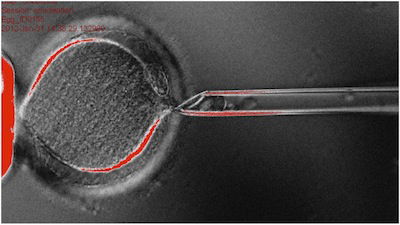
The nucleus of a human egg is removed in the first step of the cloning process. When new DNA is inserted under the proper conditions, the egg may develop into a human embryo. Image: Oregon Health & Science University www.npr.org
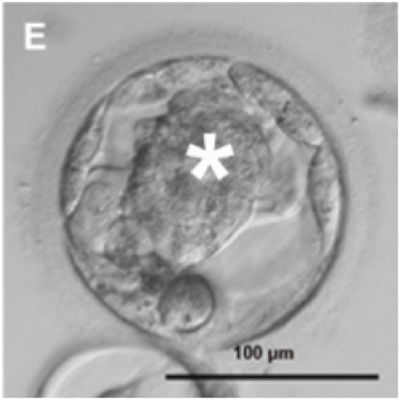
This human embryo at the blastocyst stage was produced by Mitalipov’s caffeinated cloning breakthrough. The “inner cell mass,” which is the part of an embryo that ordinarily grows to form a baby’s body, is marked with *. Embryonic stem cells (ESCs) are harvested from the blastocyst’s inner cell mass. Image: Mitalipov Lab, Oregon Health & Science University, Tachibana et al., “Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer,” Cell (2013), doi:10.1016/j.cell.2013.05.006
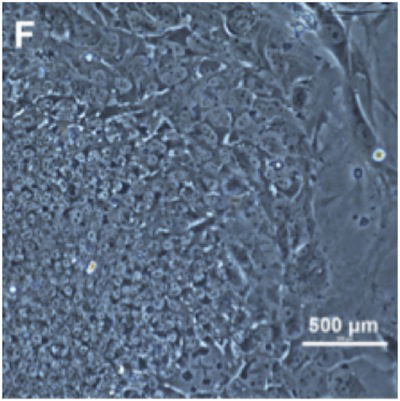
This is a culture of embryonic stem cells (ESCs) grown from the cells harvested from the now destroyed embryonic clone (E). Image: Mitalipov Lab, Oregon Health & Science University, Tachibana et al., “Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer,” Cell (2013), doi:10.1016/j.cell.2013.05.006
Background
Dolly the sheep, the first successful mammalian clone was born in 1997. Dolly started life as an embryonic clone produced by somatic cell nuclear transfer (SCNT). SCNT is the process Mitalipov uses to produce human embryonic clones. In SCNT, a donor egg’s nucleus is removed and replaced with a nucleus from a somatic cell from the animal or person to be cloned. (Somatic cells are cells like skin cells, heart cells, and muscle cells, in contrast to germ cells, which are sperm and eggs.) The fused cell is then stimulated to become an embryo—in essence, the original egg must “think” it has been fertilized, and then it behaves as a fertilized ovum does. This embryo follows the developmental blueprint in the new DNA and is therefore an embryo of the cloned organism. This method has successfully produced sheep, cows, pigs, mice, and other animals.
Human eggs (oocytes) subjected to SCNT have proven considerably more difficult to stimulate to become embryos. In 2004, Korean researcher Woo Suk Hwang fraudulently claimed to have produced human embryos by cloning as a source for embryonic stem cells. Mitalipov’s laboratory has apparently achieved what the Korean researchers only pretended to have done.2
Mitalipov’s group worked out the technical difficulties in replacing the nuclei of fragile human eggs and in then tricking them to become embryos without the stimulus of being fertilized. The initial human embryos were inferior to those obtained through in vitro fertilization. Then the group discovered the secret of success, described by another stem cell scientist as “the Starbucks experiment.”3
Perked-up Process
Mitalipov’s perfect recipe for human clones relies on caffeine. Caffeine does more than just attract people to coffee and bees to flowers. Caffeine is also a protein phosphatase inhibitor. A caffeine bath helped the egg maintain meiotic arrest during manipulation, enucleation and insertion of the new nucleus. This stabilizing effect more than doubled the likelihood of the egg with its inserted human genome responding successfully to stimulation and progressing to the multicellular stage know as a blastocyst. The addition of caffeine greatly improved the quality of the human embryos, making them indistinguishable from those produced through in vitro fertilization.4
The human embryos produced by Mitalipov’s recipe would be unable to establish a viable pregnancy because the cells needed to form the placental attachment to a mother don’t function properly. Experimental cloned monkey embryos also had this problem and, despite over a hundred attempts, were unable to successfully implant to produce viable pregnancies in monkey trials.5 (No attempt was made to use these human embryos for reproduction.) These embryonic clones are considered “therapeutic clones”—the term applied to clones developed to harvest embryonic stem cells—rather than “reproductive clones” for that reason. (Obviously there is nothing “therapeutic” here from the standpoint of the clones. “Therapeutic” applies to the hoped-for medical uses of embryonic stem cells.)
Mitalipov’s group has managed to get 23% of the caffeine-treated embryonic clones to develop into blastocysts of which half produced embryonic stem cells. The cells to be cloned were obtained from the skin of a fetus with a recognizable chromosomal abnormality, so it is certain the embryos were genuine clones, genetically identical “twins” of the cloned fetus. In animal research, young animals are more easily cloned than adults, so the researchers will later try the method with cells from an adult, but they expect the method to still be successful.6
“This is a huge scientific advance,” comments Dr. George Daley, director of stem cell research at Children’s Hospital Boston. “But it’s going to, I think, raise the specter of controversy again.”7
Failure To Implant = Ethically Off-the-hook?
Mitalipov does not think cloned human embryos should raise bioethical concerns. They are always created without fertilization by sperm, and those produced using this method can’t even implant successfully in a uterus. Embryos left over from in vitro fertilization (the ordinary and highly controversial targets for embryonic stem cell harvesting) are, as in natural reproduction, produced through the union of an egg and sperm from parents. But these cloned embryos are instead produced using an egg and DNA from the person to be cloned. Mitalipov says, “The procedures we developed actually are very efficient to make stem cells, but it’s unlikely that this will be very useful for [any] kind of reproductive cloning.”8
Others note—and we at Answers in Genesis agree—that even these human embryos have a moral standing as human beings. Therefore, the fact that no one—at the moment—is using these embryos to grow children is irrelevant. For instance, bioethicist and professor of medicine at the University of Chicago, Dr. Daniel Sulmasy, commenting on the cloning success, said, “This is a case in which one is deliberately setting out to create a human being for the sole purpose of destroying that human being. I'm of the school that thinks that that’s morally wrong no matter how much good could come of it. This raises serious problems because it is the first actual human cloning. We already know there are people out there who are itching to be able to be the first to bring a cloned human being to birth. And I think it’s going to happen.”9
Human life begins when a cell with a human genome, equipped to grow as an embryo, is created. Even though Mitalipov’s embryos do not originate from the union of a sperm and egg, the fact that they develop into blastocysts demonstrates they are true embryos. When this procedure was carried out with sheep cells, the result was a blastocyst that eventually became Dolly.
Dolly was a genetically identical copy of an adult sheep. The very fact that the sheep embryo, created without sperm through cloning and then grown in a surrogate sheep’s womb, obviously developed into a sheep proved the essential “sheep-ness” of the cloned embryo. Dolly’s developer, Dr. Ian Wilmut, “insisted that the fact that Dolly was cloned did not change her sheep-ness; if an embryo has a sheep’s genome, it is a sheep.”10 Logically, therefore, the embryos created in Mitalipov’s laboratory—though the cells for placental development were abnormal—were as human as those left over from IVF procedures.
Creating and destroying human lives in a petri dish in order to harvest their stem cells has the same moral implications whether those embryonic human lives were created with sperm or not. Whether that embryo is created in the laboratory or in a human fallopian tube, whether that embryo is produced by the union of sperm and egg or by insertion of a human genome into an empty oocyte, or even whether that embryo has some sort of abnormalities (as do many naturally conceived babies)—that embryo is still a human life that we cannot ethically, morally, and biblically ignore.
Human embryos are being created and destroyed using Mitalipov’s recipe. And once the procedure is refined, human embryos—whether reproductively capable or not—will be created to die on a regular basis in order to provide embryonic stem cells (ESCs). Yet experts maintain that the need for personalized, genetically matched human stem cells can be met at far less cost with induced pluripotent stem cells (iPSCs).11
Ignoring the Viable Alternatives and Accumulating More Victims
Adult stem cells have produced a number of promising therapeutic results, the growing list including cardiac disease, multiple sclerosis, leukemia, Parkinson’s disease, cirrhosis, and spinal cord injury.12 And iPSCs—which have been developed more recently—are promising. The developer of the technique to induce cells to return to their pluripotent state has even been awarded the Nobel Prize.13 ESCs on the other hand have not fulfilled the glowing therapeutic expectations accorded them.
Induced pluripotent stem cells (iPSCs) are produced without destroying any embryos. Neither are any human donor eggs required. They are produced from a patient’s own cells. iPSCs thus combine the ethical advantages of adult stem cells with the advantages of cloned embryonic stem cells. iPSCs are produced by stimulating a patient’s own cells to revert to their more “embryonic” pluripotent behavior; then they are induced to form the kind of cell the patient needs for treatment. Because cloned ESCs, like iPSCs, would be programmed by a patient’s own DNA, researchers have hoped treatments developed using them would not be rejected by the immune system.
Induced pluripotent stem cells thus already offer the same advantages as cloned embryonic stem cells but do not require destruction of embryos. Focusing resources on iPSCs instead of clone development not only avoids the current ethical problem of destroying embryos but also the future ethical danger that successful cloning technology may lead to “fetal farming” as a source of organ donation. Reliance on iPSCs in place of developing cloning technology to supply stem cells also avoids the cost of human eggs ($3000 to $7000 per “donor”) and the danger that women providing the eggs may be exploited.
Donating eggs is not like donating blood. The process of obtaining human eggs is not risk-free, being accompanied by the dangers of ovarian hyperstimulation, the possibility of injury during transvaginal aspiration of the eggs, potential delayed side effects of the intense hormonal treatments required (even cancer), and the risk of death.14 Many fear that low-income women and altruistic college students may become additional victims in human cloning research.
Harvard’s Dr. Daley says, “There may be advantages to SCNT-ES cells, but this must be rigorously proven.” And practically speaking, he says, producing iPSCs “remains considerably easier.”15 Even from a purely pragmatic and materialistic point of view, there seems to be little reason to experiment with human cloning.
Nevertheless, since ESCs from clones will now be available, Daley points out that future research will likely focus on comparing iPSCs to ESCs from the new human embryonic clones. Embryonic stem cells, in experimental animals, have had a tendency to form tumors, but iPSCs can also behave abnormally. Robin Lovell-Badge of London’s Nation Institute for Medical Research, echoing Daley, comments, “It would be really nice to derive and compare iPS cells and cells made using the Dolly technique from the same individual to see which were most normal.”16
More Cloning Conundrums and Conclusions
The bioethics problems attending this technology should be paramount. Just because something can be done does not mean it should be done. The laws of some countries, such as Canada, prohibit human cloning. Some differentiate between reproductive and “therapeutic” cloning. The United States government—unable to resolve the issue of whether to make a distinction between reproductive clones and those created for stem cell production—currently has no laws prohibiting cloning, though some states do. The European Union prohibits the patenting of embryonic stem cell lines, in effect circumventing the profit motive for the destruction of human embryos. Though their resolutions are non-binding, the European Union and the United Nations both oppose human cloning due to concerns about exploitation and violation of human dignity should reproductive cloning ever become a reality.
What of those who will claim, like Mitalipov, that these particular clones, because they are not equipped with normal placenta-forming cells able to implant and produce a viable pregnancy, have no moral relevance? After all, the last reported successful attempt at human cloning produced embryos “handicapped” by a triploid genome—three sets of chromosomes instead of two. Those embryos were equivalent to genetically abnormal embryos possessing lethal abnormalities. Progress in human cloning research has been a continuum. Beginning with a clone possessing an extra set of chromosomes, researchers progressed to the current success with an ordinary human diploid clone with a trophoblastic (placental) defect. We don’t know what is next on the horizon in cloning technology, but reproductively capable clones are probably coming. But whether human clones are produced as a source of ESCs or as source of fetal parts or as a source of cloned children, they are still human.
While the likelihood of producing healthy cloned children in the near future is low, given the numerous health problems to which cloned animals seem prone,17 the specter of cloned human fetuses being grown to provide spare parts for their cellular counterparts is horrible to contemplate. The United Nations and the European Union are rightly cautious and concerned about the future of people born through cloning. The U.S. president’s council on bioethics has warned that such “made to order” people would be treated as mere “commodities in the marketplace,” bereft of human dignity.18 Who, for instance, would have the just authority to direct the upbringing of cloned people? The person whose genetic pattern was cloned to make them? The egg supplier? The surrogate mother who carries the clone in her uterus? Those who placed the order for them and paid for their production? The scientists who create them? The government? At least when legal battles are fought over the destiny or custody of IVF embryos, those who contributed the egg and sperm are recognized as the responsible parents who can make decisions on their children's behalf. Who would represent the interests of the clones? If people are ever produced through cloning technology, they should certainly be entitled to full human rights and protected from exploitation. But our concern needs to start here—by demanding that human embryos not be created in order to be killed in the first place. Not to harvest their stem cells. And not to, in future, harvest their organs. Because human life is a continuum, from the standpoint of the sanctity of human life, there really is no difference.
Far above the philosophies of any manmade institutions and any laudable medical goals is the fact that God created human beings in His own image. “God created man in His own image; in the image of God He created him; male and female He created them” (Genesis 1:27). Human embryos—regardless of their origin—should be off-limits for the destructive harvesting of their stem cells. Creating human embryos in order to kill them is not justifiable for any reason, and research requiring stem cells with embryonic behavior can be conducted without controversy using iPSCs. Bottom line: these human clones are created in order to die, so their creation and their destruction is wrong.
Further Reading
- When Does Life Begin?
- The Debate over Stem Cells
- Stem Cell Breakthrough
- Stem Cells
- See “Human Clones: Created To Die” for more on the ethical issues with therapeutic cloning.
Footnotes
- Rob Stein, “Scientists Clone Human Embryos to Make Stem Cells,” NPR, May 15, 2013, http://www.npr.org/sections/health-shots/2013/05/15/183916891/scientists-clone-human-embryos-to-make-stem-cells.
- A few days after the publication of Mitalipov’s work in Cell, it came to light that duplicate and incorrectly labeled photographs—those documenting the fact that ESCs from clones are identical to those from IVF embryos—appeared in the paper. Since the 2004 fraud used such a ploy, the new work has fallen under some suspicion. It appears at this point, however, to have been an honest mistake on the part of the authors. See Gretchen Vogel and Jennifer Couzin-Frankel, “Mislabeled Images Bedevil Landmark Cloning Paper,” Science, May 23, 2013, http://www.sciencemag.org/news/2013/05/mislabeled-images-bedevil-landmark-cloning-paper.
- Stein, “Scientists Clone Human Embryos to Make Stem Cells.”
- Gretchen Vogel, “Human Stem Cells From Cloning, Finally,” Science 340, no. 6134 (May 17, 2013): 795, doi:10.1126/science.340.6134.795; and Masahito Tachibana et al. “Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer,” Cell 153, no. 6 (May 15, 2013): 1228–1238, doi:10.1016/j.cell.2013.05.006.
- Ibid.
- See Vogel, “Human Stem Cells From Cloning, Finally” and Tachibana et al. “Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer.”
- Stein, “Scientists Clone Human Embryos to Make Stem Cells.”
- Ibid.
- Ibid.
- Joseph Panno, Stem Cell Research: Medical Applications and Ethical Controversy (New York: Facts On File, Incorporated, 2005), 76.
- Vogel, “Human Stem Cells From Cloning, Finally.”
- “Benefit of Adult Stem Cells for Human Patients,” Stem Cell Research Facts, http://www.stemcellresearchfacts.org/treatment-list/; and Katie Moisse, “Stem Cells: New Hope for Heart Failure Patients,” November 14, 2011, http://abcnews.go.com/Health/HeartFailureNews/stem-cells-improve-heart-function-heart-failure-patients/story?id=14934467.
- Wesley J. Smith, “The Coming Public Conflict over Human Cloning,” First Things (blog), January 11, 2013, https://www.firstthings.com/web-exclusives/2013/01/the-coming-public-conflict-over-human-cloning.
- Learn more about the risks of egg donation in Mary Ann Toman-Miller, “Panelists Discuss Egg Donor Risks,” The Stanford Daily, May 2, 2012, http://www.stanforddaily.com/2012/05/02/panelists-discuss-egg-donor-risks/.
- Vogel, “Human Stem Cells From Cloning, Finally.”
- Andy Coghlan, “Human Stem Cells Made Using Dolly Cloning Technique,” New Scientist, May 15, 2013, https://www.newscientist.com/article/mg21829174.200-human-stem-cells-made-using-dolly-cloning-technique/.
- “Factsheet on Animal Cloning,” The Humane Society of the United States, http://www.humanesociety.org/issues/cloning/qa/questions_answers.html.
- Smith, “The Coming Public Conflict over Human Cloning.”

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis

