
Early Development of the Fruit Fly as Evidence of Design
Abstract
All man-made objects reveal forethought and planning—design. The same is true for all living creatures; the hallmarks of design are clearly evident. Brief examination of the early stages of fruit fly development reveal that the anterior-posterior and dorsal-ventral axes of each generation of flies are established by the mother even before the egg is fertilized and laid. This is done by setting up gradients of different maternal factors such as proteins and mRNAs within the oocyte (egg). Nuclei exposed to different concentrations of factors along the gradients will then express different genes leading to the formation of various structures such as antennae, wings, legs, etc. While the fly is in the larval stages, it sets aside small clusters of cells, imaginal discs, which will form the adult structures at metamorphosis. Each step in the making of a fly reveals forethought and planning, and points us to the God of the Bible.
Flying insects were likely created on the fifth day and were, as everything, vegetarian. In this article, we will look at some of the intricate steps that God put in place to ensure the proper formation from generation to generation of one type of beneficial organism, the fruit fly, following the miraculous ex nihilo creation of their kind.

Figure 1 Drosphila melanogaster Image by André Karwath, via Wikimedia Commons
At-a-Glance
- Within the mother’s ovaries, developing oocytes (egg cells) are contained within chambers which are connected to each other by a stalk. More mature eggs will initiate the establishment of the anterior-posterior axis of younger eggs.
- The mother fly makes various proteins and mRNAs which are then transported into the developing oocyte.
- The maternal proteins and mRNAs are distributed to specific locations in the oocyte setting up anterior-to-posterior and dorsal-to-ventral gradients of these factors.
- Upon fertilization, the mRNAs are translated. The oocyte nucleus undergoes multiple rounds of mitosis to generate thousands of nuclei within a single cell.
- Translated products and other maternal proteins enter the nuclei in different proportions along the anterior–posterior and dorsal-ventral axes, and either activate or repress genes.
- Products of activated genes then serve to activate or repress succeeding sets of genes. Eventually, larvae are formed.
- As larvae develop, groups of cells are set aside in imaginal discs. These cells will give rise to adult fly structures during metamorphosis.
Note to Reader: By convention, gene names are written with lowercase, italicized letters, and protein names are capitalized and not italicized. In this way, one may distinguish between the gene and the protein product of the same name.
Introduction
Take a moment to look around you. Every man-made object, simple or complex, had its beginnings in someone’s mind. It first started as an idea, a mental construct, usually with some function(s) to perform. Then it went to the drawing board or computer where the object began to take shape. In time, the object was manufactured and used for its intended purpose(s). The same can be said for every created thing in nature: all of God’s creations had their origin in his mind before he brought them forth. While design may be seen in all living creatures as well as many nonliving objects, the early development of the fruit fly, Drosophila melanogaster (Figure 1), clearly shows that the making of a “simple” fruit fly exhibits forethought. The Lord had to predetermine where the head (rostral or anterior), the tail (caudal or posterior), the back (dorsal) and belly (ventral), all the organs, and appendages would be in the finished product. In other words, God had to envision what the final product would look like before beginning construction, then bring together in the proper juxtaposition all the pieces necessary for construction.
In this article, we will very briefly look at some of the very early stages of development in Drosophila. There is no attempt to detail all aspects, for there is not enough space or time. We will examine only the establishment of the anterior-posterior (A-P) and how molecular input from the mother fly influences the initial progression of development to the larval stage. We will also take a very brief look at imaginal discs and their function in forming the mature adult fly. See the references at the end for more detailed descriptions of Drosophila development as well as the development of other more complex organisms.
Formation of Drosophila Eggs
Adult female flies have two ovaries. The ovaries are made up of clusters of ovarioles. There may be up to 15 ovarioles per ovary. Each ovariole resembles something like beads on a string as each developing egg is contained within its own egg chamber, and several chambers are connected by a stalk (Figure 2). Initiation of egg formation begins in the germarium, which is the beginning “bead” at the anterior end of an ovariole. Each chamber along this string of beads contains 16 cells: 15 nurse cells and one oocyte (Figures 3a and b). A stem cell in the germarium begins the process by undergoing mitosis; however, the two daughter cells retain a cytoplasmic connection, called a ring canal, whereby they can communicate with each other. Mitosis continues until a group of cells buds off from the germarium as a cyst. The cyst remains connected to the germarium via a stalk. Eventually, 16 cells are formed, and all retain cytoplasmic connections with one or more cells. The cell that is connected to at least four other cells becomes the oocyte while the other 15 cells become nurse cells. Retention of ring canals is very important, as we will see later. The follicle cells that surround the nurse cells and oocyte are somatic cells from the mother’s gonads, yet they play a very important role in helping to set up the A-P and D-V (dorsal-ventral) axes of the developing egg and thus the embryo.
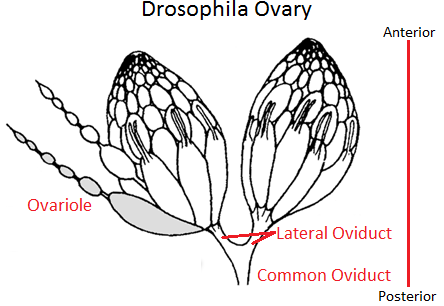
Figure 2. Drosophila Ovaries showing ovarioles, egg chambers and connecting stalks. The germarium (not labeled) is at the very tip of an ovariole. Note the “beads-on-a-string” appearance of an ovariole on the left. Image by SreeBot, via Wikimedia Commons
In Drosophila, because the egg chambers (beads) are each connected by a stalk (string), the more mature or older chamber dictates which end of the younger chamber will become the posterior and thus sets up the initial A-P polarity of the younger egg chamber.
Development of the Stalk and Positioning of the Oocyte and Oocyte Nucleus
Before proceeding further, it is important to note that many signaling molecules and their receptors are used, and it is easy to not fully appreciate the complexity of signal transduction. When it binds its receptor, the signaling molecule induces in the receiving cell a complex cascade of events in the cell, leading to a variety of changes on the cells chemistry and morphology. Each of these changes also require many different proteins working together to bring about the desired outcome.
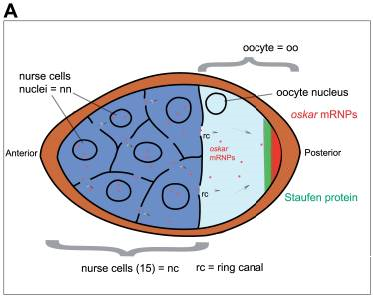
Figure 3a. Egg Chamber with Nurse Cells and Oocyte. The figure also shows the early distribution of oskar mRNA (mRNP is mRNA plus associated proteins) in the posterior of the oocyte, and another maternal-effect protein, Staufen, also located in the posterior. Later Staufen also becomes localized in the anterior as well. Image by Sanador2.0, via Wikimedia Commons
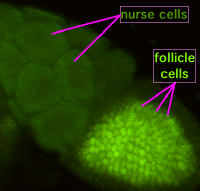
Figure 3b. Photograph of a Drosophila egg chamber with the nurse cells and follicle cells surrounding the oocyte labelled. Image by Superborsuk, via Wikimedia Commons
The oocyte in the more mature egg chamber (the last bead on the string) produces and secretes a signaling protein called Delta. Delta binds to its receptor on the anterior follicle cells of its egg chamber and induces these cells to become specialized anterior polar (border) cells. The anterior polar cells then produce and secrete a different signal protein, Unpaired, which binds to its receptor and induces adjacent anterior follicle cells to divide and generate a stalk joining the next youngest egg chamber; the stalk connects the anterior of the older chamber with the posterior of the younger chamber (the string between two beads).
In an egg chamber, the oocyte is initially surrounded by nurse cells, but then moves to the posterior end of its egg chamber where it comes into contact with posterior follicles cells. In response to Unpaired from stalk cells and Gurken from the oocyte nucleus. These follicle cells increase production of special adhesion molecules on their surfaces so that when the oocyte migrates to the posterior of its egg chamber, it is held there by these adhesion molecules. Placement of the oocyte in the posterior of its egg chamber is the first step in delineating the A-P axis of the next generation of flies. In short, the more mature egg chamber exerts an influence on the less mature egg chamber by specifying the posterior end of the younger chamber, thus ensuring that all the “beads” have their oocytes at the same end. What we see here is that even before the egg is fully formed, fertilized, and laid, the mother fly is establishing the body axis of her offspring.
The oocyte nucleus also becomes localized in the posterior of the oocyte by movement on microtubules. Here it transcribes and releases gurken mRNA only into the region of the oocyte between the nucleus and the posterior follicle cells. The mRNA is translated into protein, creating a locally high concentration of Gurken in the posterior of the oocyte. Gurken diffuses to the posterior follicle cells, binds to receptors in those cells, and induces them to produce another signaling molecule that flows back to the oocyte and induces a reorganization of the microtubules so that they will transport the oocyte nucleus and other molecules anteriorly. Gurken plus Unpaired proteins induce the posterior follicle cells to become specialized and play a role in development of the extreme posterior end of the embryo, the Telson. The nucleus moves toward the anterior end of the oocyte not in a straight line, but along the future dorsal side of the embryo. As the nucleus moves, gurken mRNA is translated, and Gurken protein diffuses to and influences the follicle cells in proximity to the future dorsal cells of the embryo. This is the initial event in determining the D-V axis.
A Gradient of Maternal Factors in the Oocyte Establish the Anterior-Posterior Axis
Maternal factors are mRNAs and proteins. They are called maternal-effect gene products because they affect aspects of early development of the embryo, but they arise from the mother’s cells, not the fertilized egg. They are initially deposited by nurse cells into the anterior of the oocyte and then transported on microtubules to the posterior of the oocyte. Two of these are nanos mRNA and oskar mRNA (Figure 3a). Once in the posterior of the oocyte, oskar mRNA is translated into Oskar protein. Oskar then binds nanos mRNA and aids in its transport to and localization in the oocyte posterior.
To supply the oocyte with ample maternal factors, nurse cells increase the number of chromosomes in their nucleus, become polyploid, in order to increase the production of particular mRNAs and proteins to be transported into the oocyte via the ring canals. The accumulation of these maternal factors by the oocyte leads to its great increase in size. Eventually, the oocyte occupies virtually the entire egg chamber. Thus far, there have been at least 39 maternal-effect genes identified. Five well-characterized maternal factors are bicoid, nanos, hunchback, caudal, and oskar. Messenger RNAs for each of these are made by the nurse cells, then transported into the anterior region of the oocyte. The distribution of these maternal factors divides the oocyte into three broad regions—anterior, abdominal/posterior, and terminal (Figure 4). Later, gene products arising from the fertilized egg, called zygotic gene products, further subdivide these broad regions into smaller regions, which are later subdivided again into smaller developmental regions by yet other genes of the embryo. For simplicity, we will confine this discussion to the anterior and posterior regions only, and the five maternal factors mentioned.
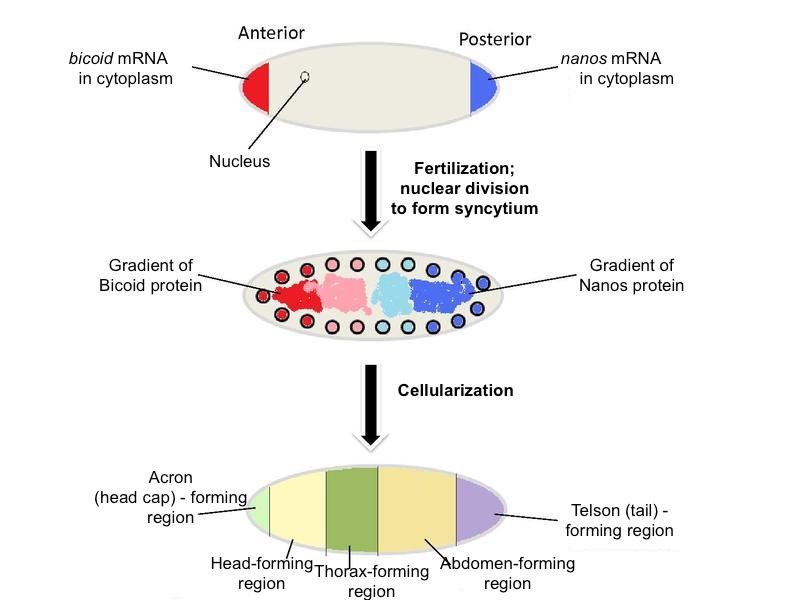
Figure 4. Distribution of nanos mRNA and bicoid mRNA (top), their protein products (middle), and the broad regions set up by these and other maternal factors. Adapted from Wikimedia Commons
The maternal mRNAs for various genes are initially deposited in the anterior of the oocyte, then transported along microtubules to the posterior along with the nucleus. After Gurken interacts with its receptor on the posterior follicle cells causing a reorganization of the microtubules, certain maternal molecules (e.g., bicoid mRNA) are transported to the anterior-most end of the oocyte where it is translated into Bicoid (Figures 4 and 5). Bicoid is essential for proper development of the head region, thus it is crucial that it be localized in the anterior region. This could only be done if the microtubules reorient in response to the signal from the posterior follicle cells, which could only signal reorientation when they receive the Gurken signal from the posteriorly located oocyte nucleus. Likewise, the oocyte itself must be located and held in the posterior of the egg chamber by special adhesion molecules induced by the signal, Unpaired, from the older egg chamber. Do these molecules “know” where they are supposed to be and when? Certainly not! They move into position according to a preconceived plan or design.
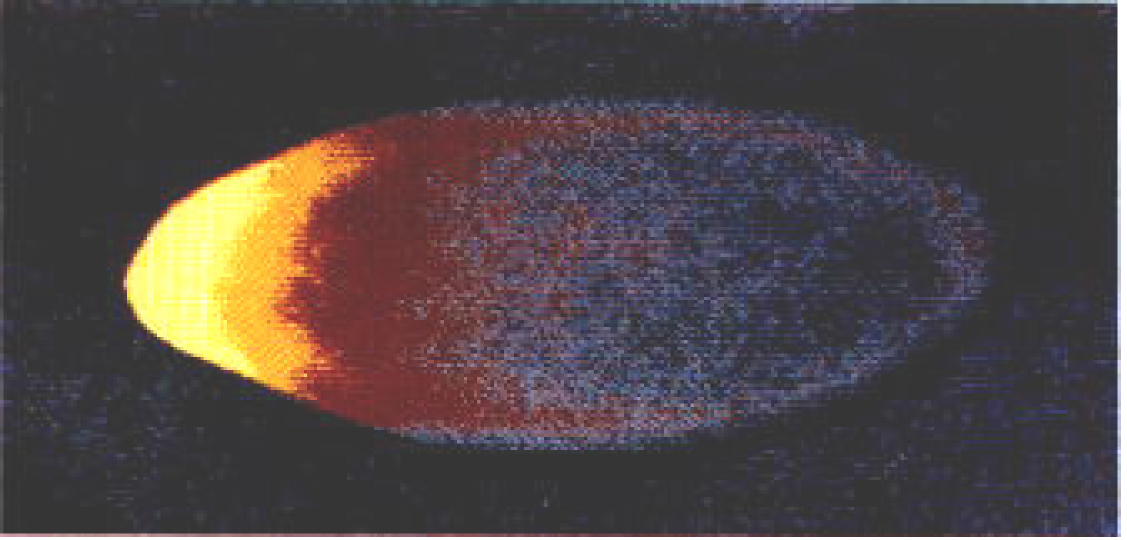
Figure 5. Bicoid Gradient from Anterior to Posterior visualized with fluorescently labeled antibodies directed toward Bicoid. Bicoid is highest in the anterior (bright yellow), but declines posteriorly. Image via Charodeika ya, via Wikimedia Commons
In the posterior part of the oocyte, oskar mRNA is translated into Oskar. Oskar is instrumental in organizing what is termed pole plasm. This is a granular region of cytoplasm that will ultimately give rise to about 16–32 pole cells, which form sperm or oocytes to produce the next generation of flies. All the ingredients necessary to make sperm or egg are localized by microtubules in the posterior portion of the oocyte even before laying and fertilization. Oskar also binds to nanos mRNA and transports it to the posterior end of the oocyte. Nanos protein is critical for proper development of the abdominal region.
We have mentioned but a few of the many maternal factors. We see that bicoid mRNA is localized in the anterior, while oskar and nanos mRNAs are in the posterior. Hunchback and caudal mRNAs are uniformly distributed, but their expression/translation into protein is restricted to either the anterior (as for Hunchback by Nanos) or posterior (as for Caudal by Bicoid) (Figures 6, 7, and 8). Upon fertilization, the mRNAs are translated into their respective protein products, creating locally high concentrations of the particular protein. Most of these proteins are transcription factors that work in the nucleus and either directly or indirectly activate or repress gene activity.
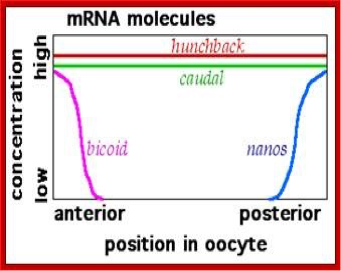
Figure 6. Distribution of mRNAs for several maternal effect genes. Image by Fred the Oyster, via Wikimedia Commons
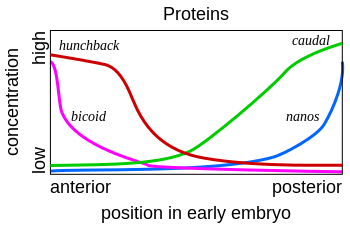
Figure 7. Protein gradients of several maternal effect genes. Note that the distribution of Bicoid and Nanos mirror their mRNA distribution, but those of Hunchback and Caudal do not. See text for the explanation. Image by Fred the Oyster, via Wikimedia Commons
Zygote Formation
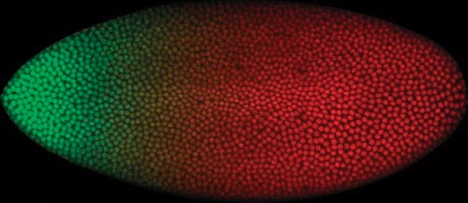
Figure 8. Bicoid (green) and Caudal (red) Gradients within the Syncitial Blastoderm. Each colored spot is a nucleus that either contains Bicoid, Caudal, or both (yellowish region of overlap). Image by Thomas Gregor, Julien O. Dubuis, Shawn C. Little, via Wikimedia Commons
Shortly after the sperm enters the oocyte in the anterior region and the two pronuclei fuse together, the newly formed zygotic nucleus begins to undergo a series of mitotic divisions to produce thousands of nuclei. It must be stressed that only the nuclei undergo mitosis, not the fertilized egg cell, as there is no cytokinesis. This type of cleavage pattern is common to insects and is referred to as superficial cleavage. It results in the formation of a syncytial blastoderm, a single cell with thousands of nuclei. It is essential that there is only one cell because, when the various maternal mRNAs are translated, their protein products must have easy access to the nuclei where they enter and have their effects on gene activity. The proteins enter the nuclei via nuclear pores and bind to certain regulatory gene sequences or other proteins on genes. All this must happen within a short time period—within two hours post-fertilization. Once cell membranes form around the nuclei, the maternal factors no longer have their effect. Essentially, we have thousands of nuclei embedded in a “soup” of maternal factors—but the maternal factors are distributed in a particular pattern from anterior to posterior.
While the nuclei are going through mitosis, many of the maternal mRNAs are being translated to create short-lived, locally high concentrations of the protein products. Because the mRNAs have certain distribution patterns in the oocyte, their products often have the same patterns (Figures 6 and 7). Bicoid activates translation of maternal hunchback mRNA in the anterior, and Nanos inhibits its translation in the posterior, creating an anterior to posterior gradient of Hunchback. Thus, we find the highest concentrations of Bicoid and Hunchback in the anterior region, and the highest concentrations of Nanos and Oskar in the posterior.
Different genes respond to differing concentrations and proportions (or ratios) of maternal factors. For example, a nucleus located in the anterior region of the egg will be exposed to a high concentration of Bicoid and Hunchback, while a nucleus located more posteriorly will be exposed to a lower concentration of Bicoid and possibly no Hunchback. As one proceeds from anterior to posterior, different nuclei will be exposed to different proportions of all the various maternal factors; any given nucleus along the A-P axis will be exposed to a particular ratio of transcription factors. The specific ratio of factors determines which genes are activated and which are repressed in that nucleus. The picture very quickly becomes quite complicated when one considers that there are many more maternal factors in play than just the few mentioned here, each with its own particular distribution pattern.
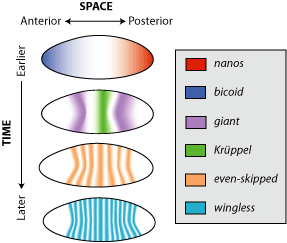
Figure 9. Distribution pattern for maternal effect proteins (Nanos and Bicoid), and the zygotic genes: gap genes (giant and kruppel), a pair-rule gene (even-skipped), and a segment polarity gene (wingless). Note the diffuse pattern of Bicoid and Nanos and the gap proteins, Giant and Kruppel, but not the pair-rule and segment polarity proteins. The latter are expressed after cellularization and thus affect only cells in which they are made. Image by Debivort, via Wikimedia Commons
After about three hours post-fertilization, the nuclei have migrated to the periphery of the egg, and cell membranes begin to form, forming the cellular blastoderm. The gradients of maternal factor proteins function to activate the first group of zygotic genes, called Gap genes. From this time forward, only zygotic genes are active. Gap genes are activated just prior to cellularization. Gap gene activation delineates subdivisions within the broad regions set up by the maternal factors, Bicoid and Nanos (Figure 9). Gap-gene protein products (e.g., Giant and Kruppel) will act by activating or repressing the next set of zygotic genes, the Pair-rule genes (e.g., Even-skipped), which in turn will influence the activity of the next set, the Segment polarity genes (e.g., wingless), and so on. In a similar fashion to the maternal proteins, Gap-gene products also form overlapping gradients mirroring regions of gap-gene activity (Figure 10). Thus, by the time cells form, all the nuclei have already received instructions as to what type of cell they will generate.
In order to get a properly formed fly, the initial maternal factors and later zygotic factors must be placed in the proper positions and ratios to activate the next set of genes in nuclei located at specific positions along the A-P axis—clearly indicating purposeful design.
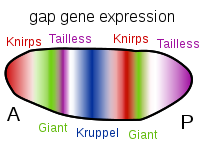
Figure 10. Patterns of Gap Gene Expression from Anterior to Posterior. Expression of each of these, and others not mentioned, is due to the specific ratio of maternal factors in a given region. These genes are activated prior to cellularization thus the diffuse banding indicating that the protein products of these genes are able to diffuse through the cytosol. This capability ends once the cellular blastoderm is formed. Image by Artoria2e5, via Wikimedia Commons
A Gradient of Maternal Factors in the Oocyte Also Establish the Dorsal-Ventral Axis
As with the development of the A-P axis, there is also a gradient of maternal factors from dorsal to ventral and ventral to dorsal. Very shortly after initiating the A-P axis, the D-V axis begins to be set up with the migration of the oocyte nucleus anteriorly adjacent to the future dorsal cells. Except for this, the two axes, A-P and D-V, are set up somewhat independently. Due to space limitations, the reader is encouraged to see any of the references below.
Imaginal Discs and the Adult Body Form
After the various gene sets have been activated along both axes, at about 24 hours post fertilization, the embryo hatches and begins the first larval stage (or instar). After about three instar stages, the larva undergoes the complex process of metamorphosis and exits as an adult fly.
During the larval stages, specific cells are set aside to form the various adult structures. These special groups of cells are called imaginal discs (Figure 11). There are 19 discs most distributed in pairs along the A-P axis of the larvae. Each disc contains from 15 to 45 cells. During metamorphosis, cells in some discs will give rise to antennae, cuticle, wings, legs, eyes, and so on. Cells in other discs will give rise to tissues of the adult abdomen. Still other discs contain cells that will give rise to the various adult organs.
The larval fly has no idea what the final fly will look like or what will be needed, yet it sets aside cells in imaginal discs that will be needed to form the adult fly. Clearly, the making of a fly proceeds along a carefully laid out plan with forethought from the Designer.
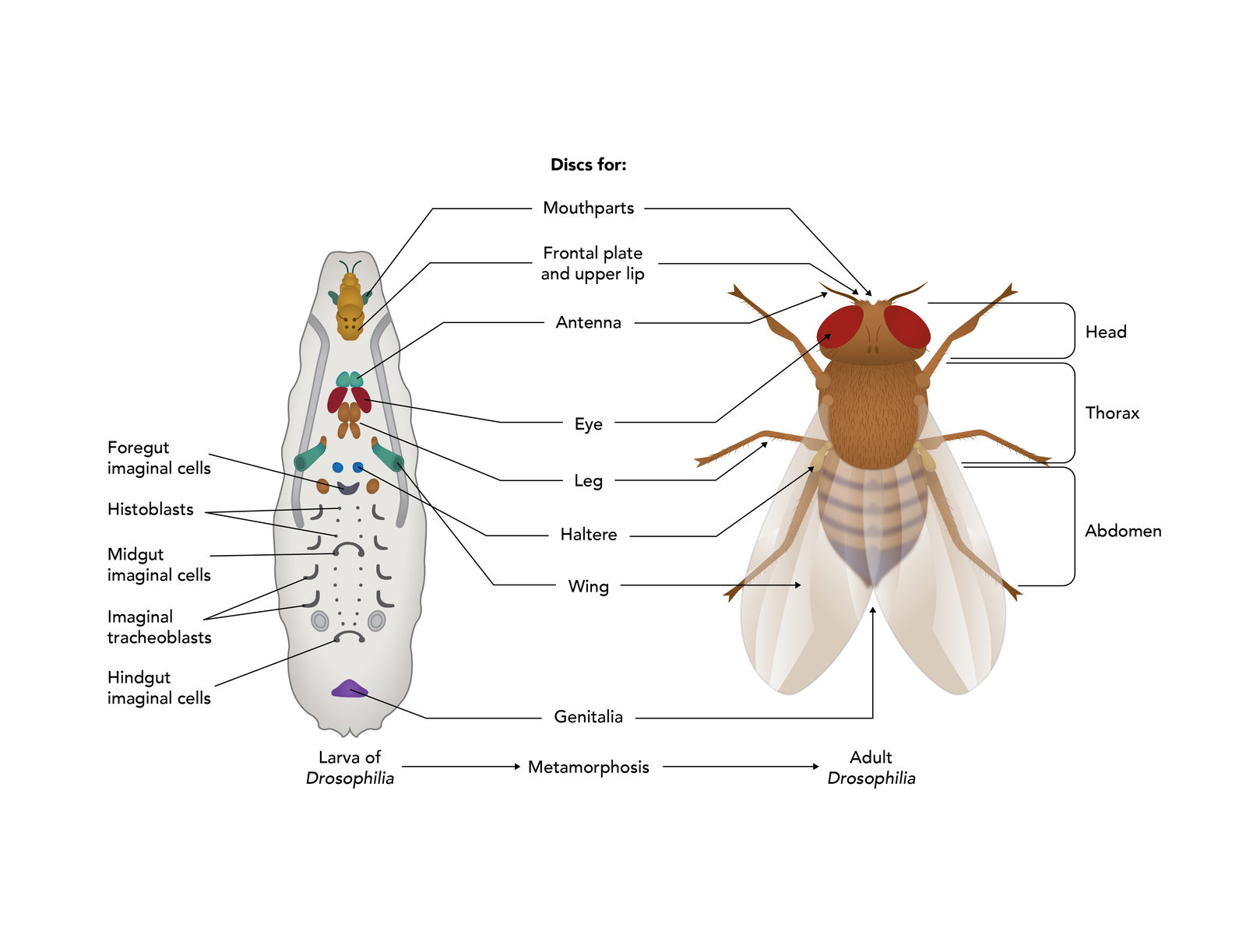
Figure 11. Isolated Imaginal Discs. Small clusters of cells set aside in the larvae to form adult structures during metamorphosis. Image by Emily Roberts, Answers in Genesis.
Conclusion
When the development of any organism is studied on the molecular and cellular level, the picture quickly becomes quite detailed and complex. However, it is in the study of life on this level that the hand of God is clearly seen; the devil is not in the details, but the God of the Bible is. However, those who are willfully ignorant fail to recognize the awesome and wondrous works of the Lord our God in creation (Romans 1:20).
References
Gilbert, Scott F. Developmental Biology, 10th ed. Sinauer, 2014.
Slack, J. M. W. Essential Developmental Biology, 3rd ed. Wiley-Blackwell, 2013.
Wilt, F.H. and Sarah C. Hake. Principles of Developmental Biology. New York: W.W. Norton & Co., 2004.
Wolpert, L., et al. Principles of Development, 5th ed. Oxford University Press, 2015.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


