Staph Bacteria from First Breath
The Interweaving of the Nasal Microbiome with the Intricate and Complex Nose
Abstract
Many microbes live in a mutualistic relationship with the human body, make up the human microbiome, and play a role in our health by stimulating and modulating the immune system. Man’s body is “covered” both inside and outside with millions of microbes that play a role in maintaining normal bodily functions and sustaining life in our changing world. The inner nose in the human body is colonized by millions of microbes during the first week of life. This internal colonization of the upper respiratory system is termed our nasal microbiome. Though we cannot see it, this microbiome is important for normal functioning, especially in a pathogenic world.
Staphylococci interact to stimulate the immune system, and play a role with the interface of the immune system, specifically generating antibody production early in life. This article focuses on possible origin of Staphylococci, their role in the nasal microbiome, the host benefit via their presence in the microbiome, and their role in creation.
Resident nose bacteria are highly diverse, and an understanding of the nasal microbiome is necessary to gain insight into microbial involvement in human health and infectious disease. The normal nasal microbiota provides clues to the pre-Fall function of bacteria. It is “normal” and critical for our body’s health to be symbiotically inhabited by microbes, such as beneficial bacteria. God’s very good creation likely included microbes in the nose and throughout the body, and these can provide clues for human health in the future.
Introduction
In Genesis 2:7, we read that God formed man out of dust from the ground and breathed into his nostrils the breath of life. Then, man came alive—a living soul! Although God mentions the human nose in Genesis 2:7 and a few other places in the Bible, very few creation articles cover it. Brian Thomas (2008) and my book, Body by Design (Gillen 2009), declare that the intricate and complex nose is wondrously designed. I assert that like other parts of the body, it too is wonderfully complex, has intelligent design, has an interwoven design, and is crafted skillfully.
Enter Staph
Within a week of creation, there is a high likelihood that some of our first breaths inhaled Staphylococci in addition to oxygen in the air. It may have not been our literal first breath, but research indicates that most infants carry Staphylococci within a week of birth. I have seen evidence of this in my own studies at Liberty University.
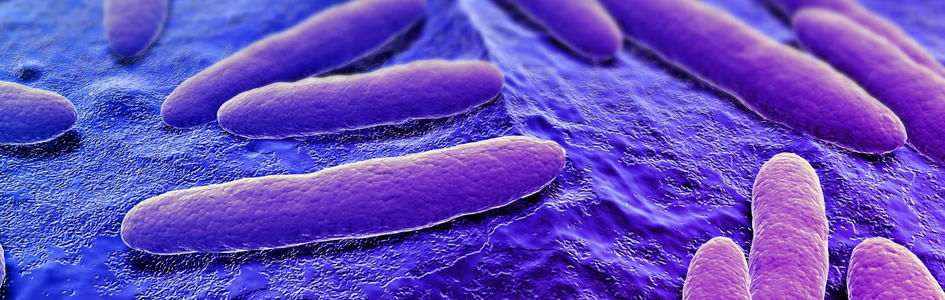
This article focuses on the Staphylococci, the nasal microbiome, its benefits, and role in creation. According to Elek (1959), there is general agreement that the main reservoir of S. aureus is the human nose. Skin surfaces harboring S. aureus (Fig. 1) become colonized from the nose, and bacteria found in wound infections come either directly or indirectly from the nose.
Figure 1. Staphylococcus aureus with golden pigment, staphyloxanthin. Image by Microrao, via Wikimedia Commons.
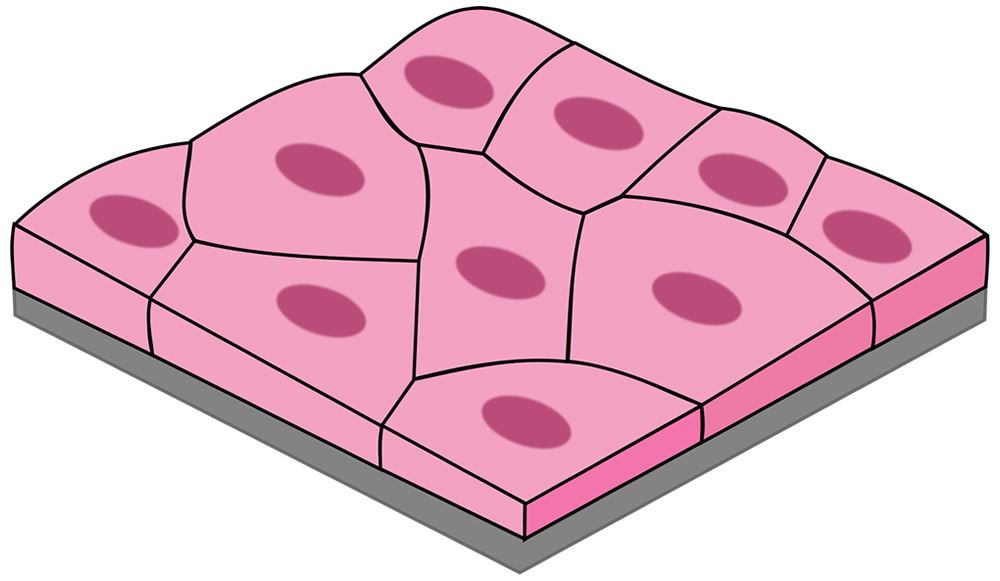
Figure 2. Simple squamous epithelial cells line the anterior nares and those first colonized by staphylococci. Image by Kamilx3, via Wikimedia Commons.
Staphylococci and micrococci of some kind are normal constituents of the nose. Studies since 1938 consistently show 32–34% of adults carry S. aureus. These results coincide with those found in our studies at Liberty University over four years. Some studies estimate that 85% of humans will be carriers at some point in their adult lives.
Bacteria are in the air—and probably have been since creation. For many, during our first breath of life, we inhaled S. aureus. According to Elek (1959), early studies of infants in England demonstrated that 96–100% were colonized by S. aureus by day 14. Some studies showed that by day three of life, 50% of infants were colonized. Phage typing showed that the strains in the infant's nose were seldom the same as those in the mother's nose. On the other hand, the Staphylococci found in the air and dust of the hospital corresponded with the strains discovered in the noses of babies and nursing staff (Elek 1959).
The actual first site of colonization is the squamous epithelium (Fig. 2) of the vestibule and multiplication of the organism occurs without invasion of tissue. Hence, I conclude that these Staphylococci were not likely pathogenic by original design. After primary colonization occurs, some of the Staphylococci move posteriorly in the nose and stimulate the ciliated, pseudostratified epithelium of the nasopharnyx (Fig. 3); this will stimulate the mucociliary mechanism. This type of symbiotic existence without invasion is very normal in infants, and the almost-universal carrier state as an infant appears to build the rest of the normal microbiota and immune system associated with the nasal passages.
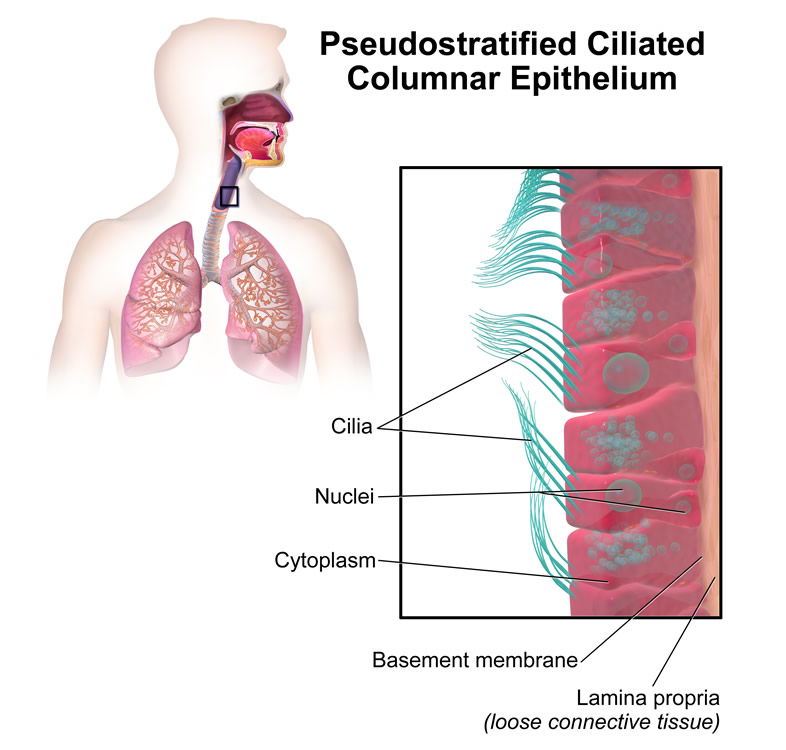
Image by BruceBlaus, via Wikimedia Commons.
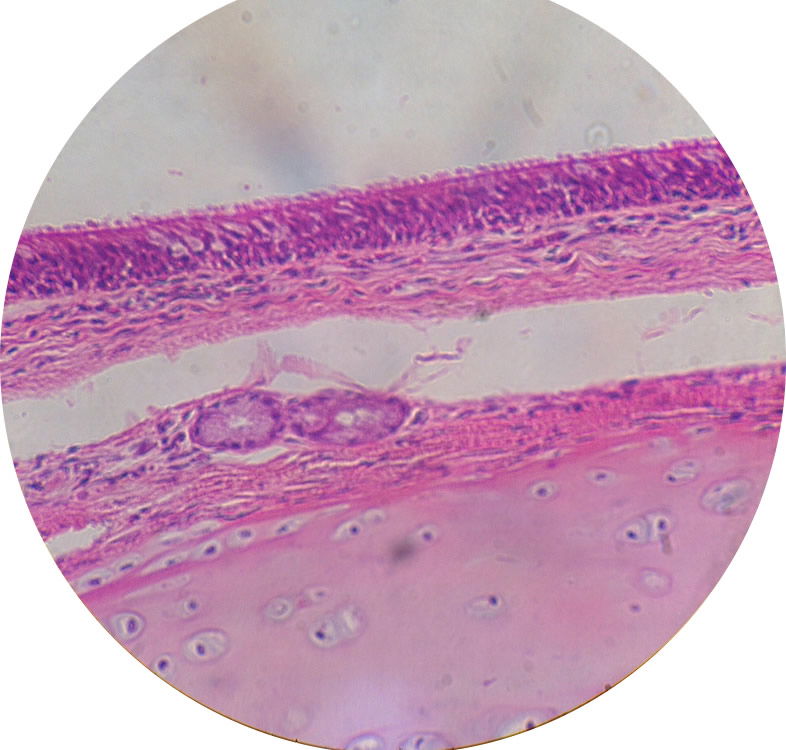
Image by Cmdrjameson, via Wikimedia Commons.
Figure 3. Ciliated, Pseudo-stratified, epithelial cells line the nasopharnyx and those last to be colonized by staphylococci.
Most species of Staphylococci (34 of 40) are generally nonpathogenic today. Even S. aureus has some strains that are non-pathogenic. Yes, I am very aware of pathogenic S. aureus strains and MRSA, as my previously published articles have shown (Gillen 2009b; Gillen, Daycock, and Serafin 2014). However, the emphasis of this article is to explain some of the “very good” design and purpose of Staphylococci and other good, beneficial bacteria of the nose.
Thank you for making me so wonderfully complex! Your workmanship is marvelous—how well I know it. You watched me as I was being formed in utter seclusion, as I was woven together in the dark of the womb. (Psalm 139:14–15 NLT)
Nose Anatomy and Physiology
The nose is one of the few openings through which bacteria have direct access to the body interior (Fig. 4). The nasal passage is important for filtering the air we inhale, as well as for impeding foreign particles and microorganisms from entering the body. The nose and nasal passages are shielded from direct sunlight, and are warm, well-oxygenated, and moist; this creates an optimum environment for some bacteria, both good and bad. To understand the physiology of the nose, its functions must be understood. The nose serves as the only means of bringing warm, humidified air into the lungs. It is the primary organ for filtering out particles in inhaled air, and it also serves to provide first-line immunologic defense by bringing this inspired air in contact with mucous-coated membranes that contain immunoglobulin A (IgA). Inspired air with bacteria is brought high into the nasal cavity to come in contact with the microbiome, thereby providing the stimuli for the production of antibodies and other enzymes, which is intimately associated with lymphatic development. Awareness of the interrelationship between the upper airways and nasal microbiome has increased. The microbiome, respiratory tract, and immune system are considered to be an integrated system, each system affecting the others. Hence, changes in the physiology of the nose and microbiota can, and will, affect the lower airways and vice versa (Marieb & Hoehn 2015). Lining the mucosa with microbes may be part of the embroidery made by the Master Craftsman at creation (Genesis 2:17).
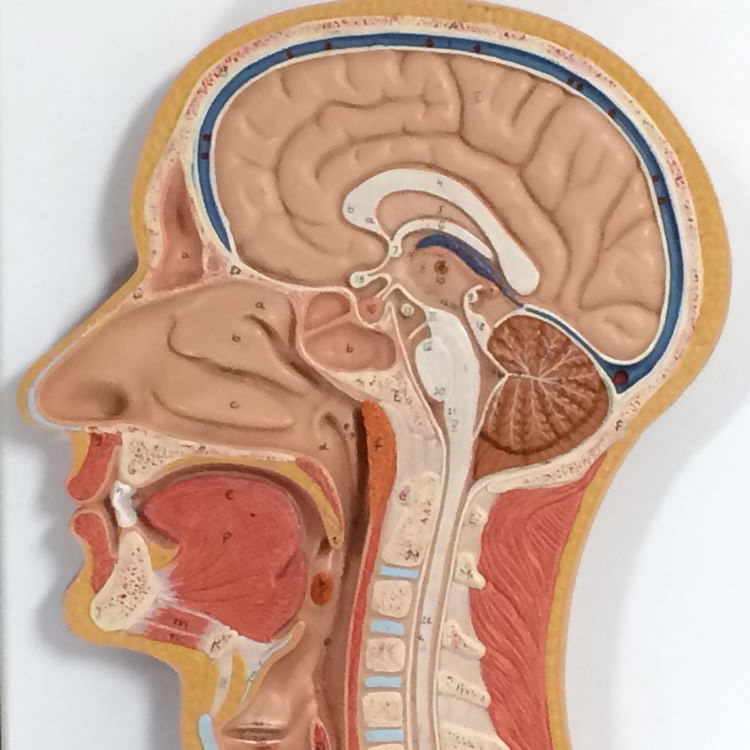
Figure 4. Respiratory Model showing the anatomy of the inner nose. Image by author.
The nasal cavity (Fig. 5) just above the nostrils is called the nasal vestibule and is lined with glands and numerous hairs. These hairs, or vibrissae (from vibro = to quiver), filter coarse particles from the inspired air. Staph colonizes the simple squamous epithelium of the anterior nare. The rest of the nasal cavity is lined with respiratory mucosa and contains pseudostratified ciliated columnar epithelium and goblet cells, which secrete a watery fluid containing enzymes. The glands daily secrete a quart of a mucous containing lysozyme, which is antibacterial, so staph rarely colonize these posterior areas of the nose. The sticky mucous traps inhaled dust, bacteria, and debris. The lysozyme attacks bacteria chemically. The epithelial respiratory mucosa also secretes defensins, natural antibiotics that get rid of invading microbes, although these defensive substances usually do not kill staphylococci. The nasal passageway walls are layered with respiratory mucous membranes. The mucous lubricating them is secreted by goblet cells. These membranes have many cilia, which move mucus in waves toward the throat area. Bacteria, along with dust and other particles inhaled from the outside environment, are snared by the nasal mucus, carried back out to the pharynx, and dripped into the gastric juices to destroy any possible pathogens. Additionally, the high content of mucous acts to humidify air (Marieb & Hoehn 2015).
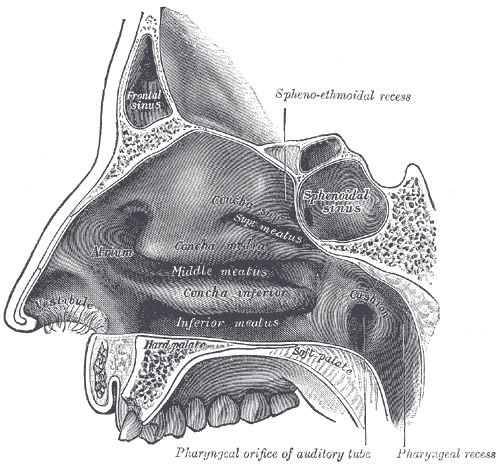
Figure 5. Anatomy of the Nose depicting the inner nasal passages and vestibule where nose hairs are first vibrated by dust and bacteria. Image by Pngbot, via Wikimedia Commons.
Wilson (2004) reported that hairs in the nostrils remove large particles present in inhaled air, while smaller particles and suspended bacteria become trapped in the mucous covering the nasal mucosa. In the posterior two thirds of the nasal mucosa, the mucociliary escalator propels the mucous-entrapped particles into the pharynx, and the whole nasal mucosa is replaced within 20 minutes. A wide range of antimicrobial agents has been detected in this fluid. Lysozyme is one of the major antimicrobial chemicals. About 75% of plasma cells of nasal mucosa are IgA producing, and 25% produce IgG. They block microbial adhesion for most bacteria. Many of the normal nasal microbiota are resistant to this antibody clearance and stay adhered to cells. The research suggests that in early life of an infant, these antibodies are not yet present in numbers. Hence why S. aureus easily adheres in early life, causing the stimulation of IgA and IgG production, and the maturation of the immune system. The role of the normal microbiota is to stimulate and mature both the second and third levels of defense in immunity (Bauman 2014). Bomar (2016) reports that this includes stimulation of lymphocytes and neutrophils (Fig. 6). These microbes were meant to interact with the immune system from the beginning. Many studies show that animals grown in axenic, or gnotobiotic (microbe-free) environments, have weaker immune systems and abnormal physiological systems.
As an infant becomes a toddler, antibody production increases. After this, only 1/3 of the population will carry S. aureus continuously for much of life. S. epidermidis (and other coagulase-negative Staph) remain for life in all humans. The 1/3 of the population that carries S. aureus for life may have an environmental base, not a genetic base. In a new study (Bomar 2016), scientists hypothesize those interactions between pathogens and other normal microbiota are an important aspect of nasal bacteria colonization. Thus, in addition to defining bacteria composition, microbiologists use bacterial counts to look for shifts in the bacterial population in the presence or absence of Streptococcus pneumoniae (pneumococcus) or S. aureus during a state of health versus disease. This knowledge is then examined for clues as to which bacteria might be interacting with each other. These clues are often in the form of positive or negative correlations of relative bacterial abundances in relation to a specific bacterium or disease. Studies by Bomar (2016), a Dartmouth research group, used this approach with a goal of understanding how benign human nasal bacteria, including Staphylococci, might protect against pneumonia bacteria—a more serious threat than MRSA most of the time.
We have observed that 100% of our students carry S. epidermidis (or other coagulase-negative bacteria, CoNS) and 33% carry S. aureus (Table 1). The probable reason why about 1/3 of our students are populated by S. aureus in the nasal passageway is due to the negative symbiotic behavior S. epidermidis and other bacteria have with S. aureus. Bacteria compete with one another for space through the synthesis of bacteriocins, bacteriolytic enzymes, etc. or the competition of specific attachment to epithelial cells. There are S. aureus strains that produce bacteriocin against some bacterium species, and some S. epidermidis strains produce bacteriocins against S. aureus (Fig. 7). The ability to bind to the epithelial cells involves the carbohydrate portion of the human nasal mucin enzyme for support. Competition for space on the mucosal surface in the nasopharyngeal region exists between bacteria as well. These bacterial species compete for limited space in order to form stable colonies and propagate.
| Section # | Number of Students | S. aureus | S. epidermidis* |
|---|---|---|---|
| 1 | 25 | 8 (32%) | 25 (100%) |
| 2 | 21 | 6 (29%) | 21 (100%) |
| 3 | 24 | 6 (25%) | 24 (100%) |
| 4 | 24 | 5 (17%) | 24 (100%) |
| 5 | 23 | 9 (39%) | 23 (100%) |
| 6 | 17 | 5 (29%) | 17 (100%) |
| 2016 Total | 117 | 39 (33.3%) | 117 (100%) |
| National Average (millions) | 33% | 100% | |
The Staphylococcus “Kind”—Family Staphylococcaceae
The Staphylococcus “kind” is a rather hardy Gram-positive bacteria type and is especially resistant to drying (Fig. 6). The first Staphylococci probably carried a diversity of genes and were able to adapt to varying microhabitats. Over time, minor changes took place (that is, variation): some of the Staphylococcus kind became S. epidermidis to occupy most of the body’s surface and nose. S. epidermidis seems quite adapted to living in both the nose and on the salty areas of the body’s surface, including specific pH environments. On the other hand, S. aureus tends to occupy moist places of the body like the anterior nares of the nose and mucous membranes and tolerates a lower pH than S. epidermidis. Perhaps the original Staphylococcus kind diversified into several varieties according to microhabitat: some to live in low pH and others for the high pH of the nose, specifically the anterior nare, nasal cavity, mucosal surface, and the nasopharnyx. S. aureus and S. epidermidis are two of 40 identified Staphylococcus species (Figs. 7–9).
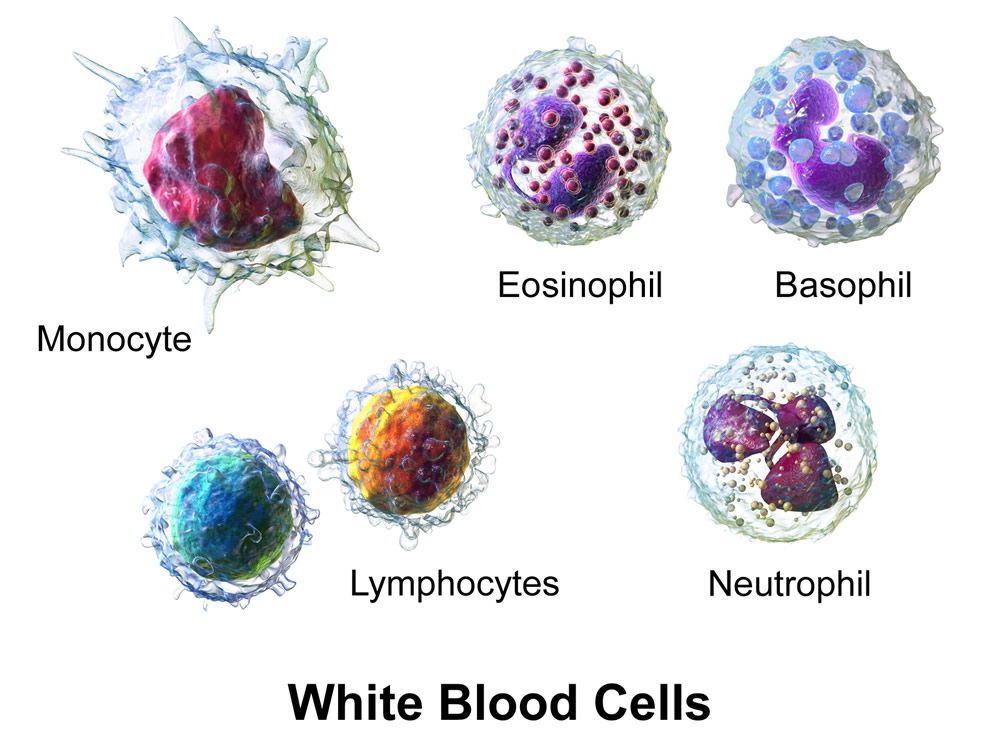
Figure 6. White blood cells. Normal microbiota commensals stimulate the immune system. Image by BruceBlaus, via Wikimedia Commons.
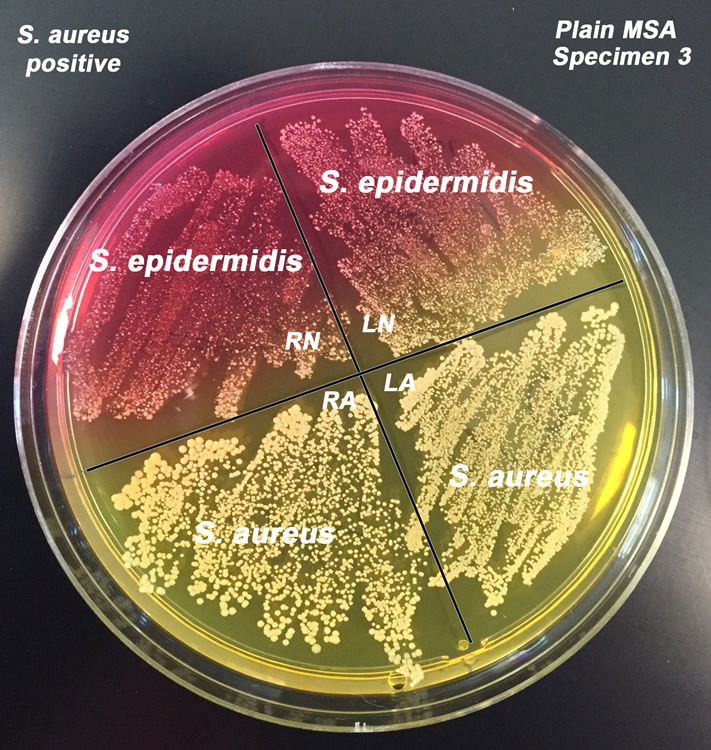
Figure 7. Staphylococcus epidermidis and S. aureus on Mannitol Salt Agar. S. epidermidis are white colonies on red background and S. aureus are gold colonies on yellow background. Image by author.
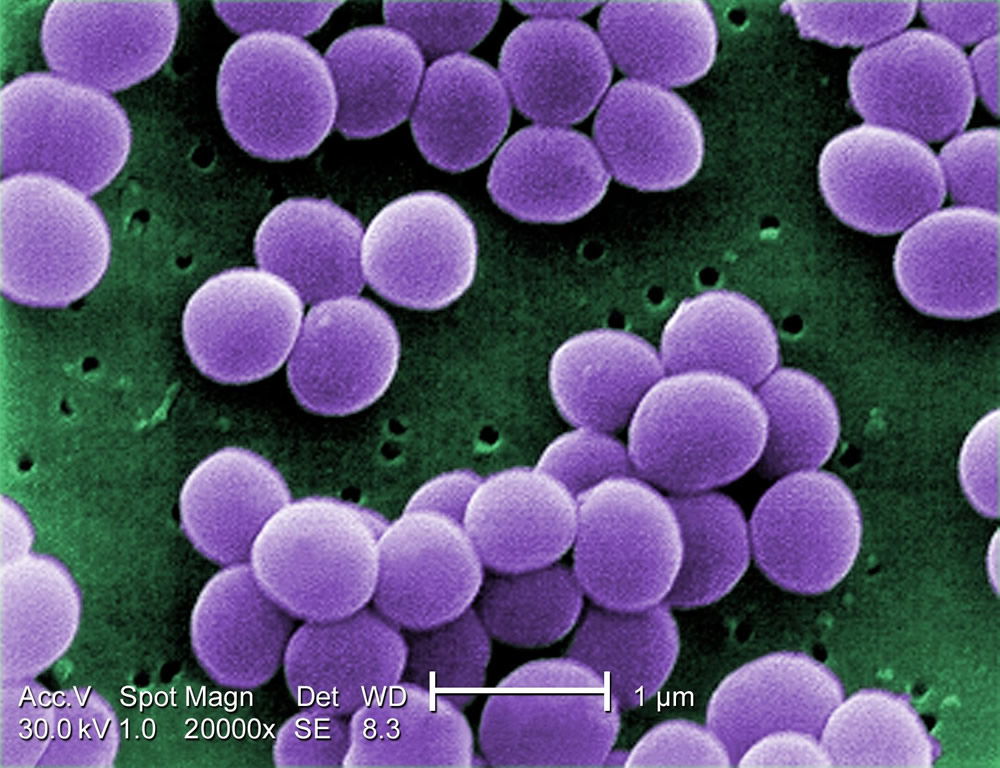
Figure 8. Staphylococcus aureus are Gram-positive cocci with a grape-like cluster formation. Image by Raeky, via Wikimedia Commons.
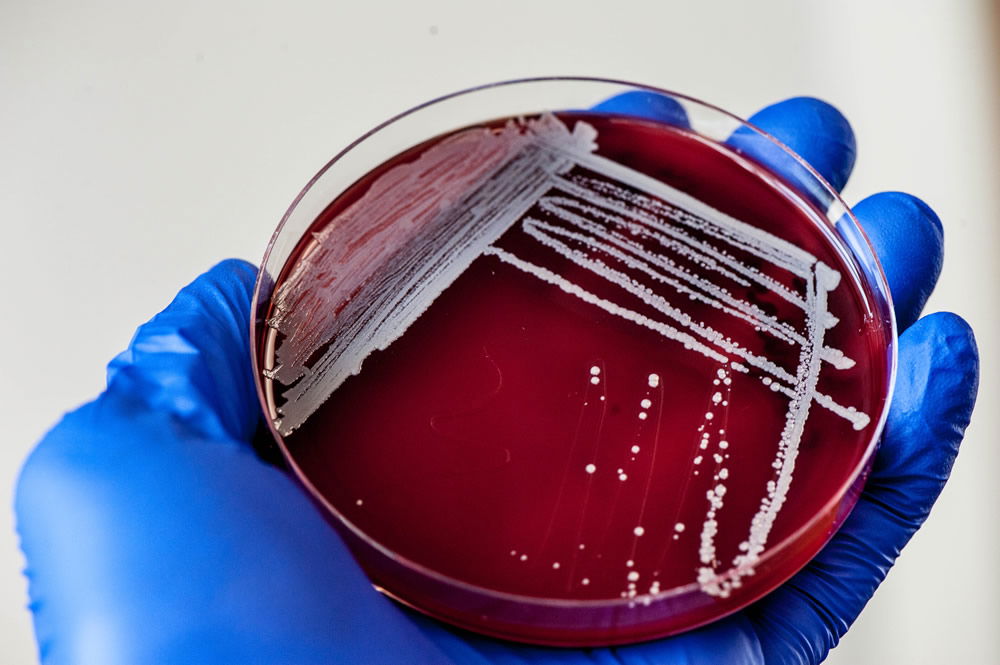
Figure 9. Staphylococci grow as white colonies on Blood Agar (5% sheep’s blood on TSA). Image by author.
At this time, over 40 species of Staphylococcus have been discovered. Many of these are found in and on the human body. While Staphylococcus is resilient and can grow at a variety of temperatures and chemical conditions, it functions very well under the conditions and temperature of the human body (Gillen 2009, 2015). S. aureus and S. epidermidis are found on the outer layers of the skin, in hair follicles, and on the nasal membranes. S. aureus are abundant in the nose, armpits (axilla), and moist areas of the body. S. epidermidis are abundant on the surface of dry skin, and sometimes are called the “skin flake” bacteria. Other types of staphylococci are also found on the conjunctiva of the eyes, the mucus membranes that line the upper respiratory tract, and the linings of the gastrointestinal and urogenital tracts. These types of Staphylococci are normally beneficial to humans, but can, at times, be harmful (Gillen 2014).
Some positive values of Staphylococcus include the ability to interact and engage the immune system and the ability to produce bacteriocins (i.e., natural antibiotics) (Tortora, Funke, and Case 2015). Bacteriocins are chemicals produced by bacteria that kill or stem the growth of a closely related type of bacteria. The varieties of Staphylococcus that are not harmful to humans when they exist solely on the skin produce bacteriocins that inhibit the existence of harmful bacteria on our skin. This symbiotic system clearly shows the hand of our loving Creator in preparing us to live in a fallen, cursed world. The Designer intelligently chose to make bacteria that keep us safe from other harmful types, and He designed it to flourish at body conditions.
From First Breath
The first breath in the life of Adam may not have had staph in it. But, it is very reasonable that as Adam breathed air over time, bacteria colonized his nose. Almost certainly, over time, Adam's descendants would breathe air that was naturally inhabited by bacteria. This microbiota is normal and natural and probably essential for the stimulation of the immune system. Most staph are inhaled from the air in our early life. The air contains dust, which is largely made of dead epithelial, skin cells, dust mites, and bacteria. Humans shed 10 billion cells per day, which is about 250 grams (1/2 pound) per year. Household dust, nurseries, and hospitals are where infants reside and pick up these bacteria early in life (Fig. 11).
Staphylococci (Fig. 8) interact to stimulate the immune system and play a role with the interface of the immune system, specifically generating antibody production early in life. The simple presence of staph bacteria after birth can change the environment in the nasal passages, if it is identified by the host's immune system.
Another key, beneficial, pediatric nasal bacteria genus is Corynebacterium. Corynebacterium accolens, a benign member of human nasal and skin microbiota, inhibits pneumococcal growth on medium supplemented with triolein. Triolein is a representative human skin triacylglycerol, similar to the triacylglycerols found on skin lining the nostrils. Like Staphylococcus, they inhibit the growth of pathogenic Streptococcus pneumoniae (Fig. 10), the most common cause of pneumonia (Bomar 2016).
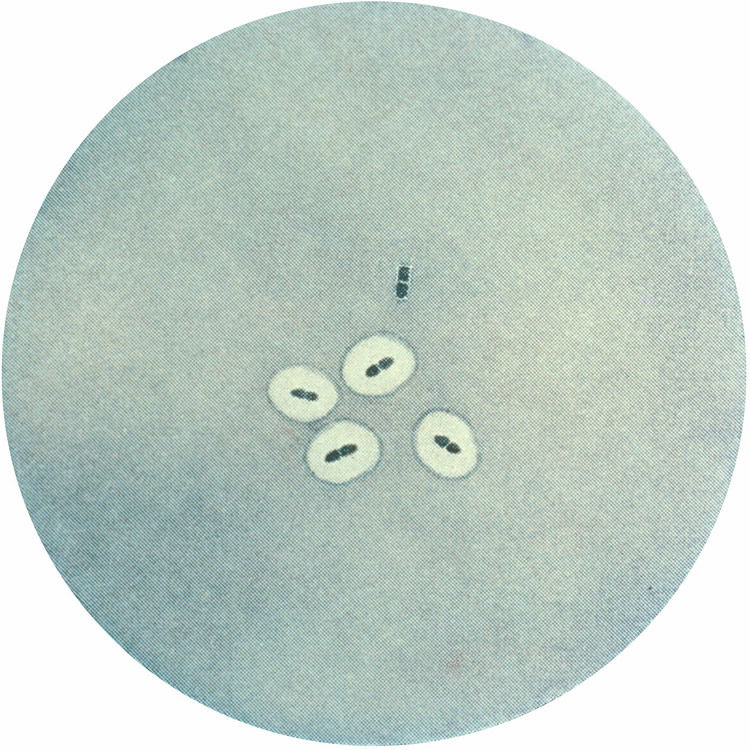
Figure 10. Streptococcus pneumoniae, the most common cause of pneumonia. Image by Splintercellguy, via Wikimedia Commons.
Pediatric Microbiome
Newborns (Fig. 11) acquire microbes from their environment, parents, siblings, and caregivers. Staph would be among the first, along with microbes acquired in the birth canal, breast milk, and food. The vast majority of environmental microbes a child is exposed to will not be acquired, because they cannot colonize the body. Either they don't have the right receptors or they can't compete with normal microbiota already present. “An important concept to remember is that of ‘selection.’ As the human microbiome becomes established, there is selection going on in multiple directions; newly encountered microbes seek an appropriate environment, which includes both the conditions at the human body site, and the microbes that are already there. At the same time, the human is selecting for microbes that provide needed services and do not cause harm. Microbes sense and interact with specific markers that are secreted by cells, or found on their surfaces, and use these cues to ‘decide’ where to grow” (Reid and Greene 2013). In any event, the set of microbes that ends up constituting a mature microbiome is far from random; it appears to be by design. During infancy (first two years of life), there is a process of adaptation between the baby and the microbes it encounters.

Figure 11. Infants in nursery. Newborns acquire microbes from their nursery environment, parents, siblings, and caregivers. Image by US National Archives bot, via Wikimedia Commons.
Research on the microbiome (Fig. 12) is fairly recent and beginning to define what constitutes a normal microbiome, how it changes over time, and how the composition and activity of the microbiome affects health and disease. The human microbiome has a profound and multifaceted effect on the body. Some may even call it “a newly recognized organ,” with a great range of metabolic activities (Reid and Greene 2013). There are normal microbiota present within both healthy and diseased individuals. Bacteria present within body systems serve to benefit the healthy host by providing functions that the body would not otherwise be capable of completing, or by occupying niches that would otherwise be occupied by pathogenic organisms. In addition to benefiting the healthy human body, the occupation of the human host serves to benefit the organisms by providing necessary resources and habitats to ensure that they survive and reproduce. Together, the normal biota and the human host interact with one another in a complex symbiosis that benefits both species (Fig. 12). This symbiosis, usually classified as mutualism or commensalism, is a key characteristic of the human species that has likely been present since Creation (Gillen 2007).
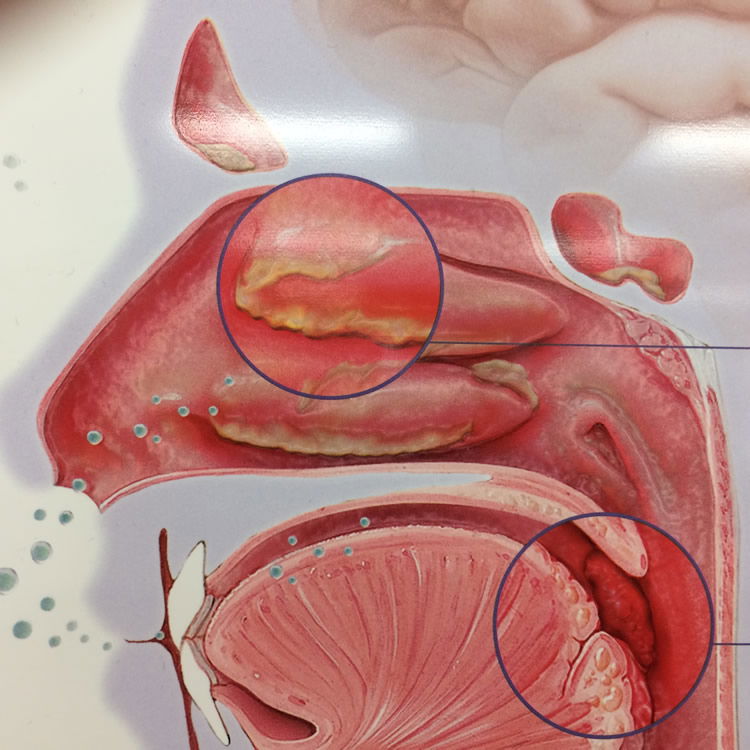
Figure 12. Normal microbiota grow on the mucous layer of the inner nose, forming a wondrous weaving of bacteria with the nose. Image by author.
Summary and Conclusions
Besides adding distinction to the human face, the nose is an amazingly complex masterpiece. The insides of our noses are not just straight hollow tubes, but are shaped specifically to regulate airflow and allow interaction with microbes. The stimulation of the immune response by the nasal microbiome is not a chance event and is a clear evidence of an intricate and creative design when systems of two independent entities work together in a seamless operation. A reasonable conclusion is that they were, and are, designed to work together. These two functions of our immune system—internal maintenance and regulation of our microbiome—involve more than just pathogen destruction but can operate for purposes other than infectious disease protection. Our noses regulate airflow direction, humidification, and immune functions by partnering with microbes and the lymphatic system. Only a wondrous designer could generate such complex features (Thomas, 2008). It appears that we are interdependent with microbial life for normal function and interwoven with a complex design even from our early days of life. The partnership between man and microbes is just beginning to be explored, but it is important to recognize that this association between Staphylococci and the nose is not random but one of intimate association made by the Triune, Creator God.
Acknowledgements
A special thanks to Rachel Walters, Andrew Worley, Rachel Ballou, Catherine Twomey, Michael Cargill, and other Liberty University students who helped with the research and photos taken in the article.
References
Bauman, R. 2014. Microbiology with Diseases by Body System. 3rd ed. San Francisco, California: Pearson Benjamin/Cummings Pub. Co.
Bomar, L. 2016. “The Nose Knows Beneficial Bacteria.” ASM News. http://www.asm.org/index.php/general-science-blog/item/3060-the-nose-knows-beneficial-bacteria.
Elek, S. D. 1959. Staphylococcus Pyogenes and Its Relation to Disease. London: Harcourt Brace/Churchill Livingstone.
Gillen, A. L. 2009a. Body by Design: Fearfully and Wonderfully Made, 6th printing. Green Forest, Arkansas: Master Books.
Gillen, A. L. 2009b. The Genesis of Methicillin-Resistant Staphylococcus aureus. Answers in Depth 4, https://answersingenesis.org/natural-selection/antibiotic-resistance/the-genesis-of-methicillin-resistant-staphylococcus-aureus/.
Gillen, A. L. 2007. The Genesis of Germs: Disease and the Coming Plagues in a Fallen World. Green Forest, Arkansas: Master Books.
Gillen, A. L. 2014. “The Wondrous Weaving of the Skin and Its Microbiome.” Answers in Depth 9, https://answersingenesis.org/human-body/wonderfully-made-design-skin-and-its-microbiome/.
Gillen, A. L. and J. Conrad. 2014. Our Impressive Immune System: More Than a Defense. Answers in Depth 9. (Posted January, 15, 2014) Answers In-Depth Article. https://answersingenesis.org/human-body/our-impressive-immune-system-more-than-a-defense/.
Gillen, A. L. and Cargill, M. 2016. “The Signature of God in Medicine and Microbiology.” Answers in Depth 11, https://answersingenesis.org/intelligent-design/signature-god-medicine-and-microbiology/.
Gillen, A., Daycock, W. and Serafin, A. 2014. “High MRSA Carriage Rate among Nursing Microbiology Students.” Advances in Microbiology 4: 871–877. doi:10.4236/aim.2014.413096.
Marieb, E. and and Hoehn, K. 2015. Human Anatomy and Physiology. 10th Ed. San Francisco: Pearson Benjamin/Cummings Pub. Co.
Reid, A. and S. Greene. 2013. Human Microbiome FAQ. Washington, DC: ASM Press.
Thomas, B. 2008. “The Amazing Design of the Human Nose.” Acts & Facts. 37 (8): 14.
Tortora, G. J., B. R. Funke, and C. L. Case. 2013. Microbiology, An Introduction, 11th ed. San Francisco, California: Pearson Benjamin/Cummings Pub. Co.
Wilson, M. 2004. Microbial Inhabitants of Humans: Their Role in Health and Disease. Cambridge: Cambridge University Press.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis