The Wonderfully Made Design of the Skin and Its Microbiome
Abstract
Many microbes live in a mutualistic relationship with the human body, make up the human microbiome, and play a role in our health by modulating the immune system. Man is “covered” inside and outside his body with millions of microbes to maintain normal bodily functions and sustain life in our changing world. The skin is the largest organ in the human body and is colonized by millions of microbes. This external colonization of the integumentary system is termed our skin microbiome. Man cannot see it (except with a microscope), but we need it for normal functioning, certainly in a pathogenic world. This article focuses on the skin microbiome, its benefits, and role in creation.
Resident skin bacteria are highly diverse, and an understanding of the skin microbiome is necessary to gain insight into microbial involvement in human skin diseases and disorders. The normal skin microbiota provides clues to the pre-Fall function of bacteria. It is “normal” and critical for our body’s health to be symbiotically inhabited by microbes such as beneficial bacteria. God’s very good creation likely included microbes on the skin, and these can provide clues for human health in the future. The skin microbiome may enable novel probiotic and antibiotic approaches.
Keywords: skin, microbiota, skin microbiome, integumentary system, pre-Fall, post-Fall, immune system, biomatrix, organosubstrate, interwoven complexity, fearfully and wonderfully made, body by design, genesis of germs
Introduction
In some recent experiments (Scott 2014), participants have eliminated using soap and spent four weeks rubbing bacteria on their skin as their daily routine. It sounds strange, yet it is appears to be working in terms of hygiene. Julia Scott was Subject 26 in an experiment using a living bacterial “skin tonic,” developed by AOBiome, a biotech start-up in Cambridge, Massachusetts. According to Scott (2014) “the tonic looks, feels and tastes like water, but each spray bottle of AO+ Refreshing Cosmetic Mist contains billions of cultivated Nitrosomonas eutropha, an ammonia-oxidizing bacteria (AOB) that is most commonly found in soil and natural waters.” Some scientists hypothesize that it once lived harmoniously with human skin as normal microbiota. This is before we started washing it away with soap and shampoo. This bacteria may serve as a built-in cleanser, deodorant, anti-inflammatory and immune booster. It metabolizes the ammonia in our sweat and converts it into nitrite and nitric oxide.
Background
The human body is carrying more than three times more bacteria than human cells. Most recent estimates of the human body cell counts are about 37 trillion and this puts the estimate of bacteria in/on the body at more than 100 trillion. The skin microbiome consists of over a trillion cells. The human skin contains about three million bacteria per cm3 (many more than the number of our own larger epidermal cells). Many Staphylococcus and Micrococcus species live on human skin and derive nutrients from the environment while producing acid to prevent the overgrowth of pathogenic organisms. S. epidermidis alone may make up 75-90% of the normal flora on the skin. The numbers alone constitute investigation of their purpose and design in human body health and disease state.
The skin provides a habitation for many bacteria (Figs. 1, 2, and 3). Dr. Joseph Francis (2003) suggests the microbes are a natural, healthy extension of the body and that bacteria form a biomatrix (i.e. organosubstrate) on the epidermis. In a brilliant stroke, the Creator most likely wove together1 man’s skin with a Staphylococcus kind. Just like an embroidered quilt, He made humans with bacteria on their skin and in their intestines. When God created man, most likely He made the Staphylococcus kind to help maintain the skin and other integumentary surfaces (Gillen 2009b).
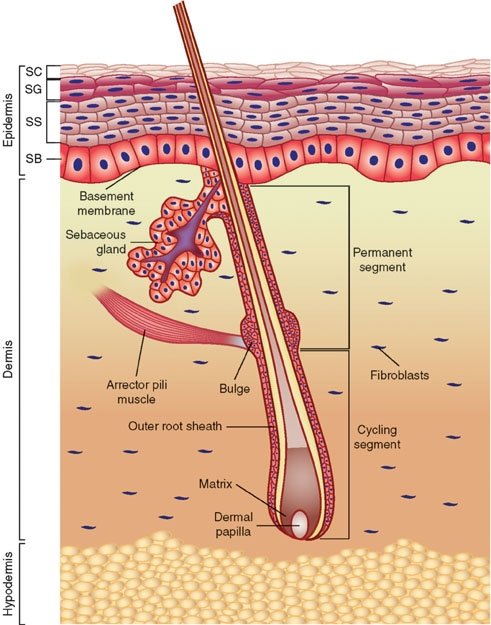 Fig. 1. Anatomy of the skin, Wikimedia commons.
Fig. 1. Anatomy of the skin, Wikimedia commons.
Over time, minor changes took place (that is, variation): some of the Staphylococcus kind became S. epidermidis to occupy most of the body’s surface. S. epidermidis seems quite adapted to living on the dry and salty skin. On the other hand, S. aureus tends to occupy moist places of the body like the nose, mucous membranes, and folds of the armpits and groin. Perhaps the original Staphylococcus kind diversified into several varieties according to microhabitat: some to live on the dry skin and some to live on moist skin. (S. aureus and S. epidermidis are two of 31 identified Staphylococcus species (Figs. 4 and 5)).
Staphylococcus aureus are bacteria that can frequently live harmlessly in the nose and occasionally on the skin in a state known as colonization. Most likely, the Staphylococcus “kind” was originally designed to live in harmony with man as beneficial normal microbiota. Perhaps it played a positive role in the recycling of cell components of the nose or skin as its “cousin” S. epidermidis did in its original good design. After the Fall, the Curse given by the Creator caused corruption and decay to the genetic code of bacteria, and pathogens emerged. A pathogen is a microbe capable of causing host damage. All pathogenic “germs” would have their origin after the Fall (Genesis 3). The Curse would have profoundly influenced all Creation, effecting pathogens and parasites. Clearly, the origin of infectious disease is complex and multifaceted. This topic is further explored in the book The Genesis of Germs (Gillen 2007), which provides some understanding into the origin of infectious diseases. From a biblical worldview, infectious diseases and pathogenesis became a secondary condition, not what the Creator would call “very good.” As research continues, the mutualistic relationship of microbes and man is seen more clearly than ever.
Awesome Anatomy and the Interwoven Skin
For You formed my inward parts; You wove me in my mother's womb. I will give thanks to You, for I am fearfully and wonderfully made; Wonderful are Your works, And my soul knows it very well. Psalm 139: 13–14 NASB.
The skin illustrates the concept of interwoven complexity (Gillen 2009a, 2009b) better than any other organ system. The skin is an organ because it consists of different tissues that are joined to perform specific activities (Fig. 2). It is the largest organ in surface area and weight, weighing twice as much as the liver or the brain. In adults, the skin covers an area of about 22-feet2 (2 m2). The skin is not just a simple, thin covering that keeps the body together and provides protection: it varies in thickness from 1/16 inch on the soles of your feet to 1/500 inch on your eyelids. Structurally, the skin consists of three principal parts: the outer, thinner portion, which is composed of epithelium, called the epidermis (Figs. 1 and 9); the middle, thicker, connective tissue part, called the dermis. Below this is third innermost layer, the subcutaneous layer, or hypodermis layer.
When Job wrote, “Thou hast clothed me with skin and flesh . . .,” perhaps he was thinking of the protection and the beauty that the Creator had provided him with skin surrounding this entire body.
The skin’s various nerves, glands and structures intertwine to produce the integumentary network below the outer epidermis. In the dermis, various glands secrete waste products from the body, and sebaceous glands secrete the oil needed in lubricating and waterproofing the skin. However, even beyond all of these intricate structures, the individual skin cells demonstrate a fabric-like design. Each cell has complex stitching that binds with other cells. These tiny anchoring junctions in the cell membranes are called desmosomes. These junctions function like rivets, fastening cells together into strong epithelial sheets. Intermediate filaments are made of keratin and reinforce desmosomes.
Bacteria, fungi, parasites, and viruses cannot easily penetrate the tightly riveted cells of the skin. As long as the skin is unbroken, it is one of our most effective defenses. Normal microbiota, harmless bacteria that live on the skin, protect the body by attacking harmful bacteria that try to take up residence. The acidity of the skin also helps to ward off harmful bacteria. When bacteria break down the oil on the skin, more acid is produced and the bacteria are prevented from overcolonization by their own waste products. The skin protects the internal organs of the body against infection, injury, and harmful sunrays. It also plays an important role in the regulation of body temperature. When Job wrote, “Thine hands have made me and fashioned me together round about . . . Thou hast clothed me with skin and flesh, and hast fenced me with bones and sinews
” (Job 10: 8, 11), perhaps he was thinking of the protection and the beauty that the Creator had provided him with skin surrounding this entire body.
Epidermis: A Woven Mantle
The epidermis (Figs.1, 9, 10) is composed of five distinct strata, and protects against abrasion and ultraviolet light, produces vitamin D, and gives rise to hair, nails, and glands. The stratum corneum (Latin for “horny layer”) is the outermost layer in the epidermis, consists of dead skin cells, and has many bacteria interwoven in it. This layer has an important interaction role with the normal biota (Tortora, Funke, and Case, 2013). The other four layers (or strata) of the epidermis include: the stratum lucidum (clear/translucent layer of the palms and soles), the stratum granulosum (granular layer), the stratum spinosum (spinous layer), and the stratum basale/germinativum (basal/germinal layer).
The epidermis is the thinnest of the three layers—at most a millimeter. Like the other layers, its thickness varies over different parts of the body. Adapted for the everyday friction of gripping and walking, it is thickest on the palms of the hands and soles of the feet but thin on the eyelids, which must be light and flexible. Beneficial bacteria feed on the waxy secretions of skin cells, and then produce a moisturizing film that keeps our skin supple and prevents cracks, thus keeping out invading pathogens and illustrating the very good biomatrix of man’s skin with microbes.
Composition of the Normal Skin Microbiome
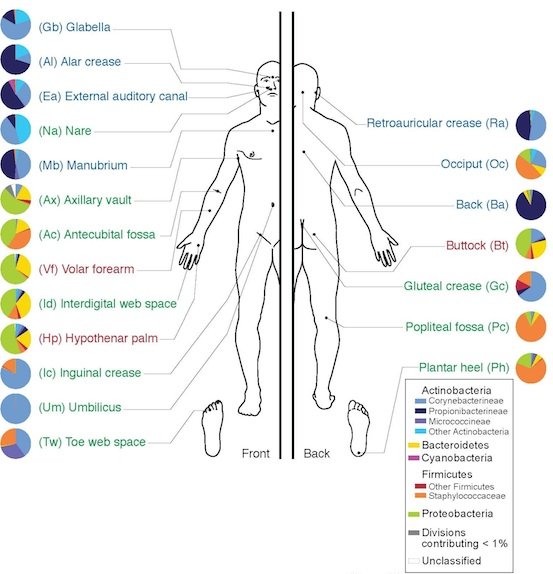 Fig. 2. Skin Microbiome, Wikimedia commons.
Fig. 2. Skin Microbiome, Wikimedia commons.
In the last several decades of scientific research, numerous discoveries have been made regarding the composition and complexity of the normal skin microbiome. Like a fingerprint, studies have shown that each individual maintains his/her own unique microbial flora on the skin. Research conducted by the Human Microbiome Project indicates that more than 2600 unique species from more than 26 different phyla are present on or within the human body (NIH 2014). The collection of bacteria, fungi, and protozoans that constitute the microbiome is not random; the human microbiome2 is made up of a particular set of microbes that complement each other and the human host.
Research on the microbiome is fairly recent and beginning to define what constitutes a normal microbiome, how it changes over time, and how the composition and activity of the microbiome affect health and disease. The human microbiome has a profound and multifaceted effect on the body. Some may even call it “a newly recognized organ,” with a great range of metabolic activities (Reid and Greene 2013). There is a normal microbiota present within both healthy and diseased individuals
Bacteria present within body systems serve to benefit the healthy host by providing functions that the body would not otherwise be capable of completing or by occupying niches that would otherwise be occupied by pathogenic organisms. In addition to benefiting the healthy human body, the occupation of the human host serves to benefit the organisms by providing necessary resources and habitats to ensure that they survive and reproduce. Together, the normal biota and the human host interact with one another in a complex symbiosis that benefits both species. This symbiosis, usually classified as mutualism or commensalism, is a key characteristic of the human species that has likely been present since Creation (Gillen 2007).
The integument of the human microbiome alone (Figs. 1, 2, and 3) is estimated to be home to up to 1000 unique species of microorganisms (NIH 20014). The normal microbiota begin to colonize the integument and body interior soon after birth, and remain present until death. While more than 90% of the skin normal flora is made up of Staphylococcus epidermidis, S. aureus, many other staphylococci, and many Micrococcus species, are present on the skin of healthy individuals.
Ecology of the Normal Skin Microbiome
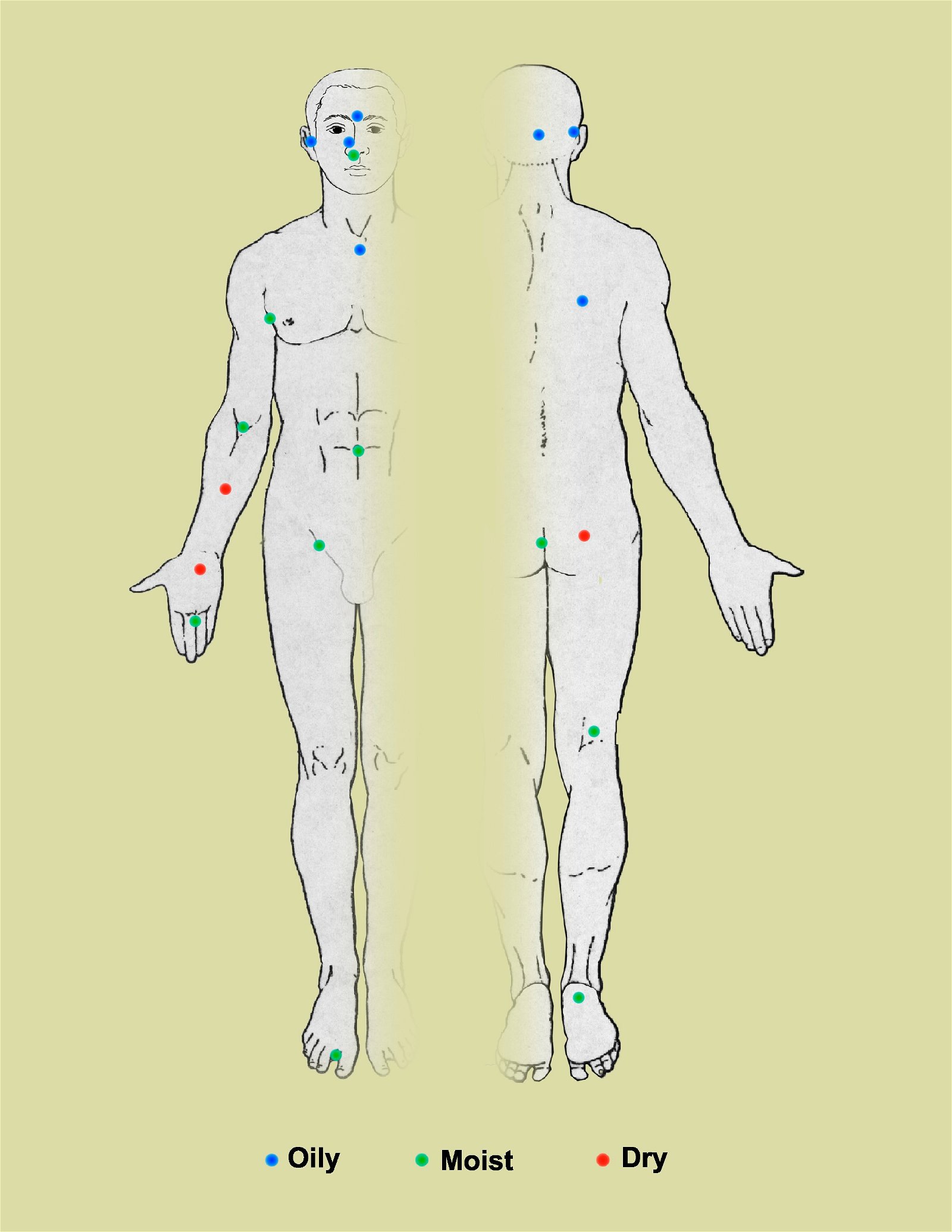 Fig. 3. Skin Microbiome and the ecology of 20 sites on the human body targeted for analysis of microbial genome sequencing, Wikipedia.
Fig. 3. Skin Microbiome and the ecology of 20 sites on the human body targeted for analysis of microbial genome sequencing, Wikipedia.
The complexity and variety of the local flora in any particular body region is dependent upon many factors. In regards to the skin microbiome, factors that determine the predominant species include nutrient levels, pH, temperature, oxygen, and water levels (Wilson 2004). According to texts (Baron, 1996; Tortora, Funke, and Case 2013), regions of the skin can be compared to the varying geographic regions of the Earth, much of it like a desert. For example, the forearm is most similar to a desert-like biome, whereas the scalp and armpits align best with the temperate forests and tropical forests, respectively. In terms of the diversity of local bacterial flora, the epidermis can be divided into three different regions. The first region includes the axilla, perineum, and toe webs, and often contains many Gram-negative bacilli in the moister areas of the skin. The second region includes the hands, face, and trunk of the body, and tends to harbor a less significant bacterial set than the areas that are high in moisture. The third set includes the upper arms and legs, and is often made up of lower numbers of microbes than the other two regions (Baron 1996).
In moist areas of the skin, Gram-negative bacteria such as Acinetobacter and Pseudomonas are found in small portions (Baron, 1996). Recent findings have also led to the discovery of a diverse scalp microbiota including various fungi and yeasts (ex. Malassezia). In addition to these, many species of the genus Propionibacterium are also present within locales on the skin that contain a large number of sebaceous glands, as well as many diptheriods, including those in the genus Corynebacterium.
The Benefits of Normal Skin Microbiota
The normal microbiota (Wilson 2004) has a symbiotic relationship with the individual. The interaction of the microbiome with the immune system may very well aid in keeping out the deleterious microorganisms that constantly compete with these beneficial bacteria. This is termed microbial antagonism: active opposition between two microbe populations. It is well known that probiotics (like bacteria in yogurt) boosts immune function, inhibit the growth of pathogens, and increase resistance to infection. Probiotics historically have been used to repopulate the large intestine with Lactobacillus and other beneficial species of bacteria. Now researchers are seeking to find probiotic bacteria that will safely and effectively colonize the surface of human skin, in order to boost the immune system and to keep pathogens and parasites out.
What would happen if these normal bacterial populations of Staphylococcus on the skin were eliminated through, for example, a high antibiotic regimen? As any dermatologist knows, fungal spores resulting in a stubborn infection would quickly occupy the vacated niche. There are several other examples of the important, beneficial work of bacteria and protozoa.
The original symbiotic function was corrupted after the Fall, and they are now pathogens.
Creation biologist Dr. Joseph Francis describes bacteria and viruses as the “organosubstrate of life” or biomatrix (Francis, 2003). From this perspective, bacteria and viruses are seen not so much as distinct organisms but as “extra-organismal organs,” that is, the means by which “higher” organisms meet their needs. Indeed, the entire ecosystem is supported by this elaborate maintenance-and-repair substrate. In the biomatrix model, Francis views the minority of disease-causing bacteria and viruses as having come about from displacement from their original created location and from modification of their biology, which may include mutation. The original symbiotic function of these organisms was corrupted after the Fall, and they are now pathogens.
It certainly appears that perhaps the immune system was originally designed to interact with the skin, gut, and elsewhere in the body (Gillen and Conrad 2014). About 10 years ago, research by Rakoff-Nahoum et. al. (2004), reported that Toll-like receptors (TLRs are proteins on the surface of sentinel cells that help the body recognize microbes) play a crucial role in host defense against microbial infection. The microbial ligands recognized by TLRs are not unique to pathogens, however: both pathogenic and good bacteria stimulate TLRs. Rakoff-Nahoum et. al. demonstrate that good, commensal bacteria are recognized by TLRs under normal steady-state conditions, and this interaction plays a crucial role in the maintenance of intestinal epithelial homeostasis.
The skin microbiota serves as an important extension of the integumentary system. The majority of microorganisms within this system occupy the superficial layers of the epidermis and occasionally branch into the dermal papilla, hair follicles, and subcutaneous sweat glands. In each of these areas, the normal bacteria occupy specific niches. These normal organisms interact with the immune and lymphatic systems to ensure that bacterial levels do not rise too high or dip too low as to cause damage to the individual. Studies show that in the absence of normal biota, bacterial infections are much more prevalent (Wilson 2004). Commensal and mutualistic bacteria on the skin induce an antibody-mediated immune response that serves to produce low levels of antibodies. These natural antibodies are capable of cross-reacting with pathogenic organisms and warding off invasion of species that do not belong on the integument. In addition to interaction with the immune system, the normal microbiota serves to produce its own agents to prevent pathogenic organisms from invading their environments. These agents include peroxides, fatty acids, and bacteriocins that are capable of inhibiting the metabolic functions and growth of other species of bacteria (Wilson 2004). This feature ensures that healthy and beneficial microbiota are the only organisms that occupy sites on the body. In a more general sense, the normal microbiota compete with pathogenic bacteria for attachment sites and essential nutrients and metabolic precursors. This is evidenced by studies that show dramatic differences in infection rates between germ-free animals and conventional animals with normal microbiota (Baron 1996).
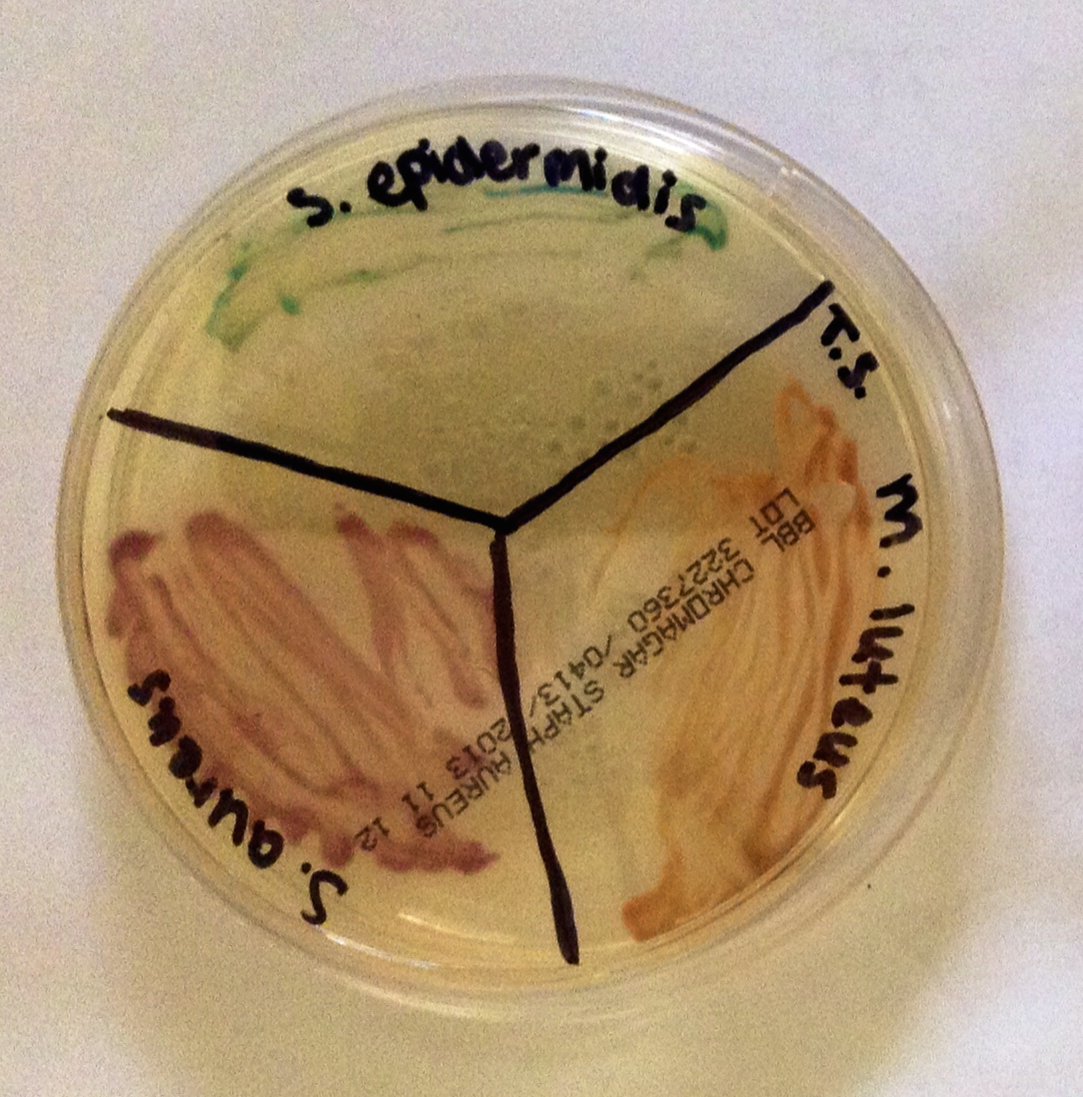 Fig. 4. Staphylococci and micrococci colonies growing on CHROMagar, Alan L. Gillen image.
Fig. 4. Staphylococci and micrococci colonies growing on CHROMagar, Alan L. Gillen image.
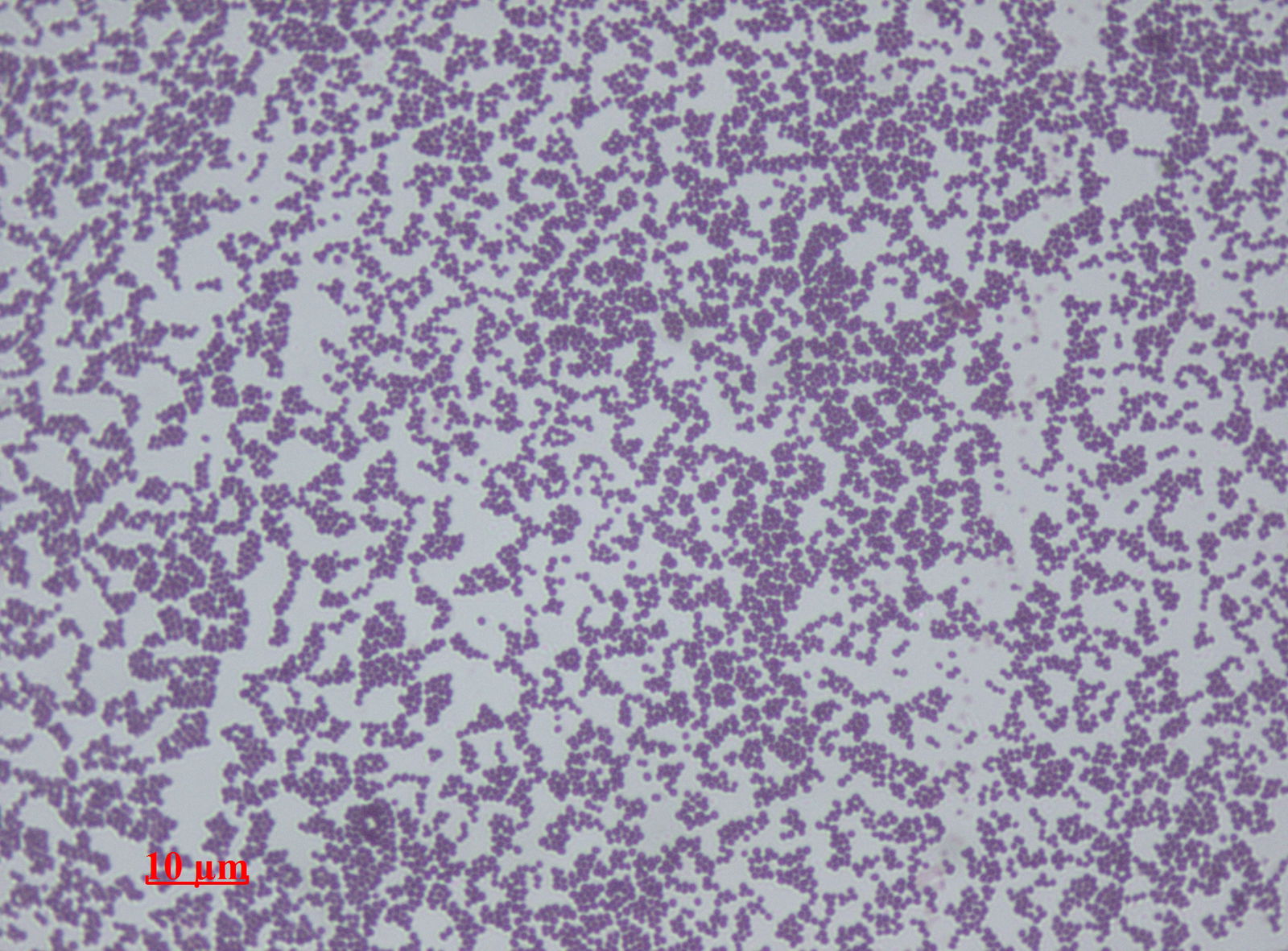 Fig. 5. Staphylococcus epidermidis, Wikimedia commons.
Fig. 5. Staphylococcus epidermidis, Wikimedia commons.
The health benefits of the normal biota were not fully understood until studies of germ-free animals became available within the scientific community. By removing animals from the womb and then maintaining them in special isolation chambers, they could remain completely absent of all normal microorganisms. These lab-created organisms had severely different features not shared with conventional animals (Wilson 2004). These differences included an underdeveloped lamina propria, a decreased renewal rate of epithelial cells, and an inability to produce proper secretions from mucous and serous glands (Baron 1996). Additionally, these uncolonized organisms lacked the ability to create an immune response against even the smallest amounts of pathogenic material. Research indicated that fewer than ten Salmonella organisms were needed to infect the germ-free animals, whereas upwards of 106 organisms are needed to infect an animal colonized with their normal microbiota (Baron 1996).
Recent studies provide a good case for the benefits we may receive from components of our skin microbiome, such as S. epidermidis (Fig. 5). In the first of these studies, Yuping Lai and co-workers (Caryl 2010) tested the hypothesis that the microbiome may serve to protect the host from unintended inflammatory diseases. They demonstrate how lipotechoic acid (LTA), a product of staphylococci, suppresses skin inflammation during wound repair, thus preventing a normal inflammatory response from becoming excessive.
Toll-like receptors (TLRs) on the surface of keratinocytes, the cells that make up your skin, are able to detect the products of bacteria. TLRs signal other cells using small molecules called cytokines, which in turn initiate a cascade of events that result in an inflammatory response. Yet S. epidermidis, which live on keratinocytes, do not initiate inflammation. The hypothesis was that S. epidermidis must be able to interfere with the signaling of TLRs, and in doing so it seems it is not only able to evade being recognized as an enemy, but it can also help decrease the magnitude of inflammation associated with skin injury.
If the skin gets wounded, the epidermal cells release their contents, and provide signals to surrounding cells that something is injured. The researchers show for the first time that RNA released from necrotic human cells triggers TLRs on neighboring healthy keratinocytes, and these in turn stimulate a proinflammatory response via production of cytokines (Caryl 2010). Caryl reports that a research team hypothesized that if S. epidermidis wants to thrive, it is in their own interest to help control excessive inflammation that may expose them to the full force of the immune system. They determined that the lipotechoic acid (LTA) in S. epidermidis produces triggers to another type of TLR on the surface of keratinocytes. These TLR-triggered cells stimulated a response in reducing the degree of inflammation in response to injury.
This is a mechanism by which a staphylococcal product suppresses inflammation. It is worth remembering, however, that whilst LTA has this unique interaction with keratinocytes, should cells of the immune system or deeper tissue detect them, it would have the opposite effect. Cells that are not exposed to the microbiome would recognize all such signals as foreign, and mount a defense accordingly. It may be possible for a probiotic therapy that helps in the moderation of inflammation with bacterial commensals at the site of such an injury for management of wound healing (Caryl 2010).
Normal Biota Prevents Heavy Staph and MRSA Infections
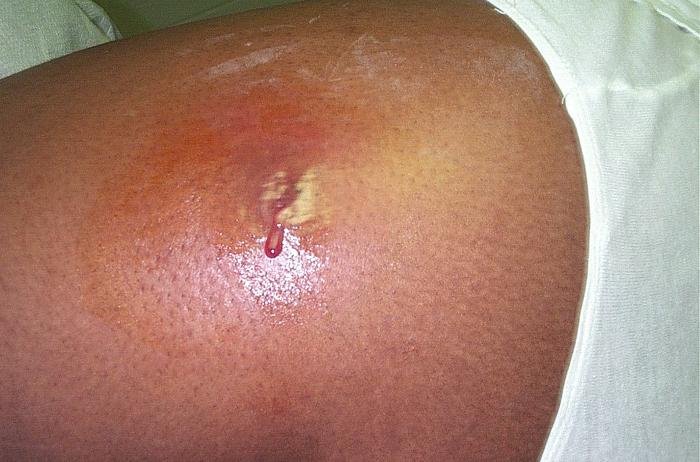 Fig. 6. MRSA Cutaneous abscess MRSA staphylococcus aureus, Wikimedia commons.
Fig. 6. MRSA Cutaneous abscess MRSA staphylococcus aureus, Wikimedia commons.
Normal microbiota prevents heavy Staphylococcus aureus and MRSA colonization and infections. “Staphylococcus epidermidis has been shown to produce compounds that inhibit the related, but pathogenic, Staphylococcus aureus.” Methicillin-resistant S. aureus (MRSA) emerged in recent years to become a leading cause of infection worldwide both in communities as well as in hospitals and clinical settings. MRSA colonization in the anterior nares (nose) and axilla (armpits) predisposes to disease and encourages transmission of the germ. However, available antiseptic soaps (ex. chlorhexidine) and topical antibiotics (ex. mupirocin) are becoming less effective at preventing MRSA colonization. Studies of human nasal microbiota suggest that resident bacteria play a critical role in limiting S. aureus growth, and prompted researchers to investigate whether application of prebiotics or probiotics (commensal resident bacteria) could prevent colonization with MRSA in the anterior nares (Fig. 6). Park, Iwase, Liu (2011) established a murine model system to study this question, and showed that mice nasally pre-colonized with S. epidermidis became more resistant to colonization with MRSA. This study suggests that application of commensal bacteria with topical antibiotics could represent a more effective strategy to prevent MRSA colonization.
Normal Biota Prevents Leishmaniasis
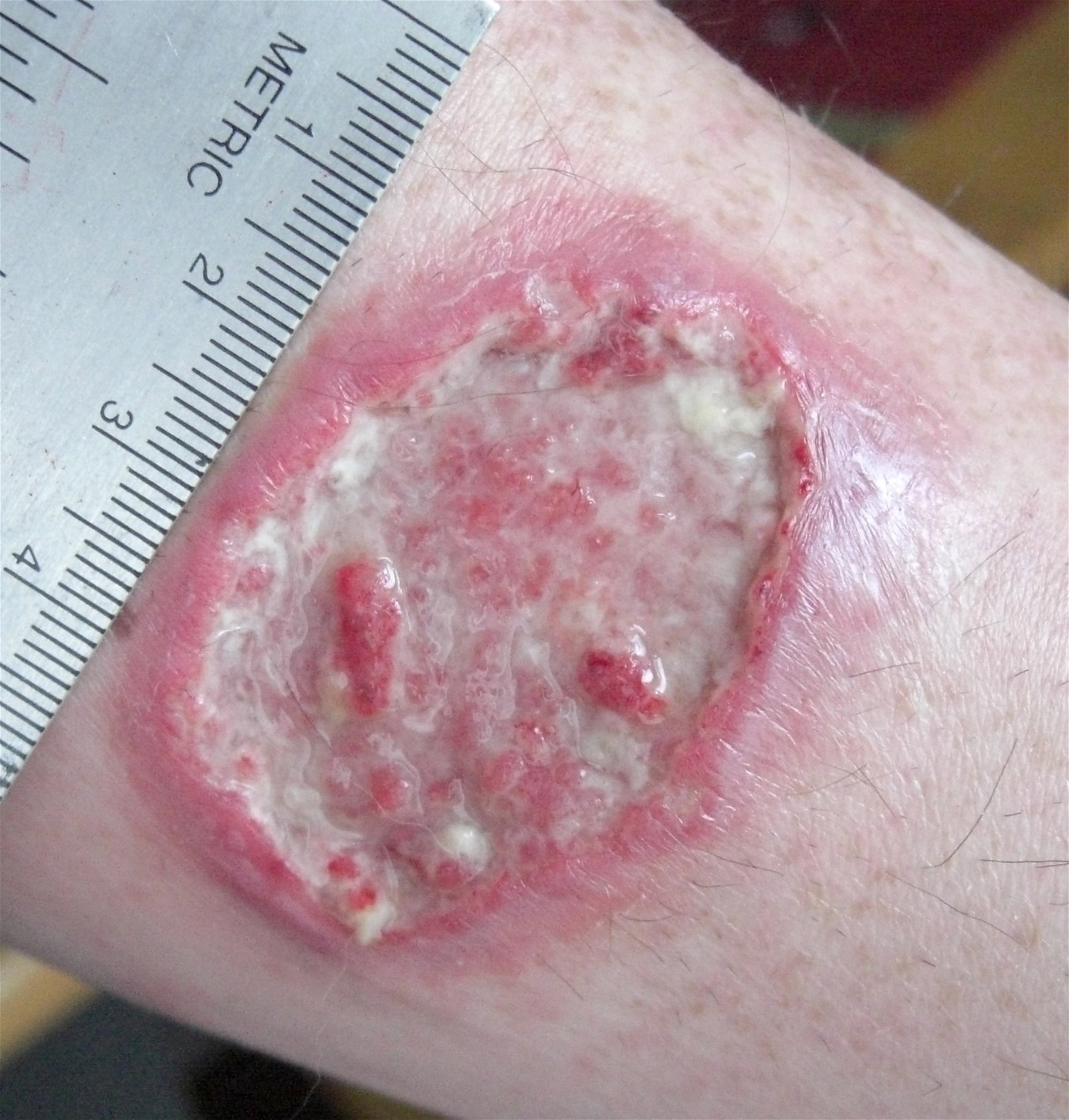 Fig. 7. Leishmaniasis ulcer. The normal microbiota protects against this Leishmaniasis (also called “White Leprosy” in some countries), Wikimedia commons.
Fig. 7. Leishmaniasis ulcer. The normal microbiota protects against this Leishmaniasis (also called “White Leprosy” in some countries), Wikimedia commons.
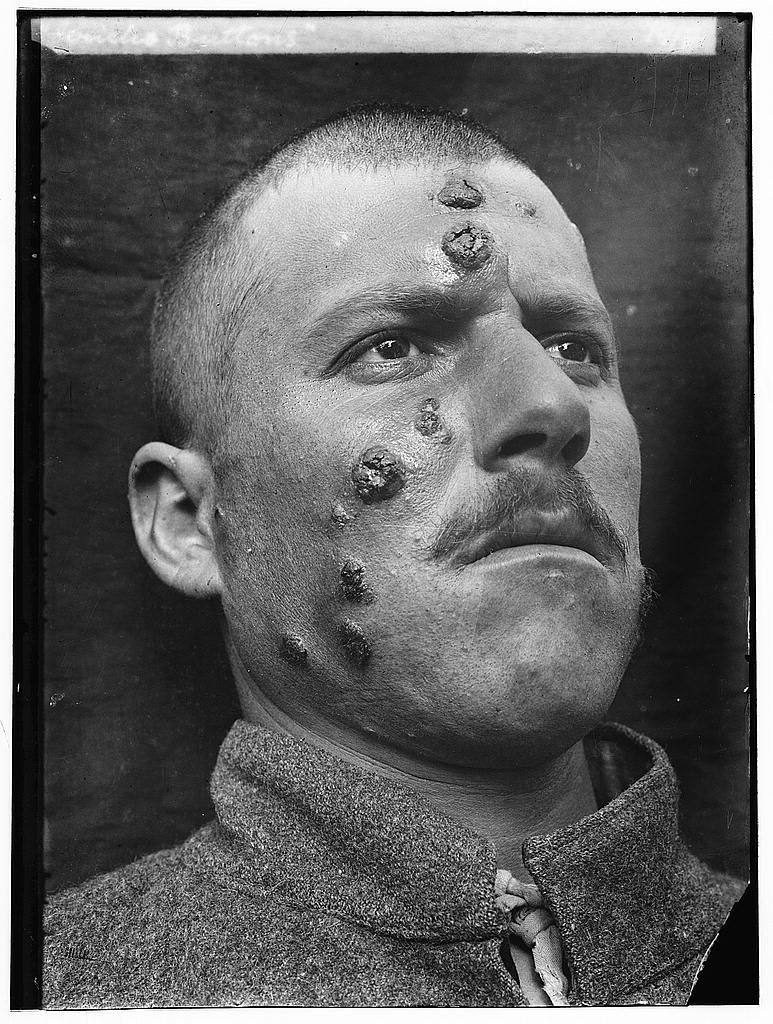 Fig. 8. This form of leishmaniasis common in the Middle East, is known then locally as "Jericho Buttons" for the number of cases near the ancient city, Wikimedia commons.
Fig. 8. This form of leishmaniasis common in the Middle East, is known then locally as "Jericho Buttons" for the number of cases near the ancient city, Wikimedia commons.
Leishmaniasis is a parasitic disease that is caused by protozoans in the genus Leishmania (Roberts, Janovy and Nadler 2013). These parasites are transmitted by the bite of infected sandflies. Cutaneous leishmaniasis is the most common form of this disease and may leave white skin lesions (Figs. 7 and 8) or patches on a person’s skin; it is sometimes called “white leprosy.” Normal microbiota prevents leishmaniasis (Naik et. al. 2012): “Using mouse models, the NIH team observed that commensals contribute to protective immunity by interacting with the immune cells in the skin.” The NIH team colonized “germ-free” mice (mice bred with no naturally occurring bacteria on the skin) with the human skin commensal Staphylococcus epidermidis. These staphylococci stimulated and “trained” the immune system to effectively defend against Leishmania parasites.
The NIH team observed that colonizing the mice with this one species of good bacteria enabled an immune cell in the mouse skin to produce a cell-signaling molecule needed to protect against harmful microbes. The NIH team subsequently infected both colonized and non-colonized germ-free mice with a parasite. Mice that were not colonized with the bacteria did not mount an effective immune response to the Leishmania parasite; mice that were colonized did. Although human experimentation has not yet begun, people in the Middle East, where this is common, could especially benefit. One cannot wonder if people near the ancient city of Jericho were plagued with leishmaniasis since the time of Joshua (Fig. 8). In this region of the world, cutaneous leishmaniasis is still common.
In separate experiments, the NIH team found that microbiota found in different tissues—skin, gut, lung—have unique roles at each site and that maintaining good health requires the presence of several different sets of commensal communities. This study (Naik et. al. 2012) provides new insights into the protective role of skin commensals and demonstrates that skin health relies on the interaction of commensals and immune cells. Further research is needed, say the authors (Naik et. al. 2012), to determine whether skin disorders such as eczema and psoriasis may be caused or exacerbated by an imbalance of “good” skin bacteria and potentially harmful microbes that influence the skin and its immune cells.
Intricate Design and Wondrous Weaving of the Normal Skin Microbiome
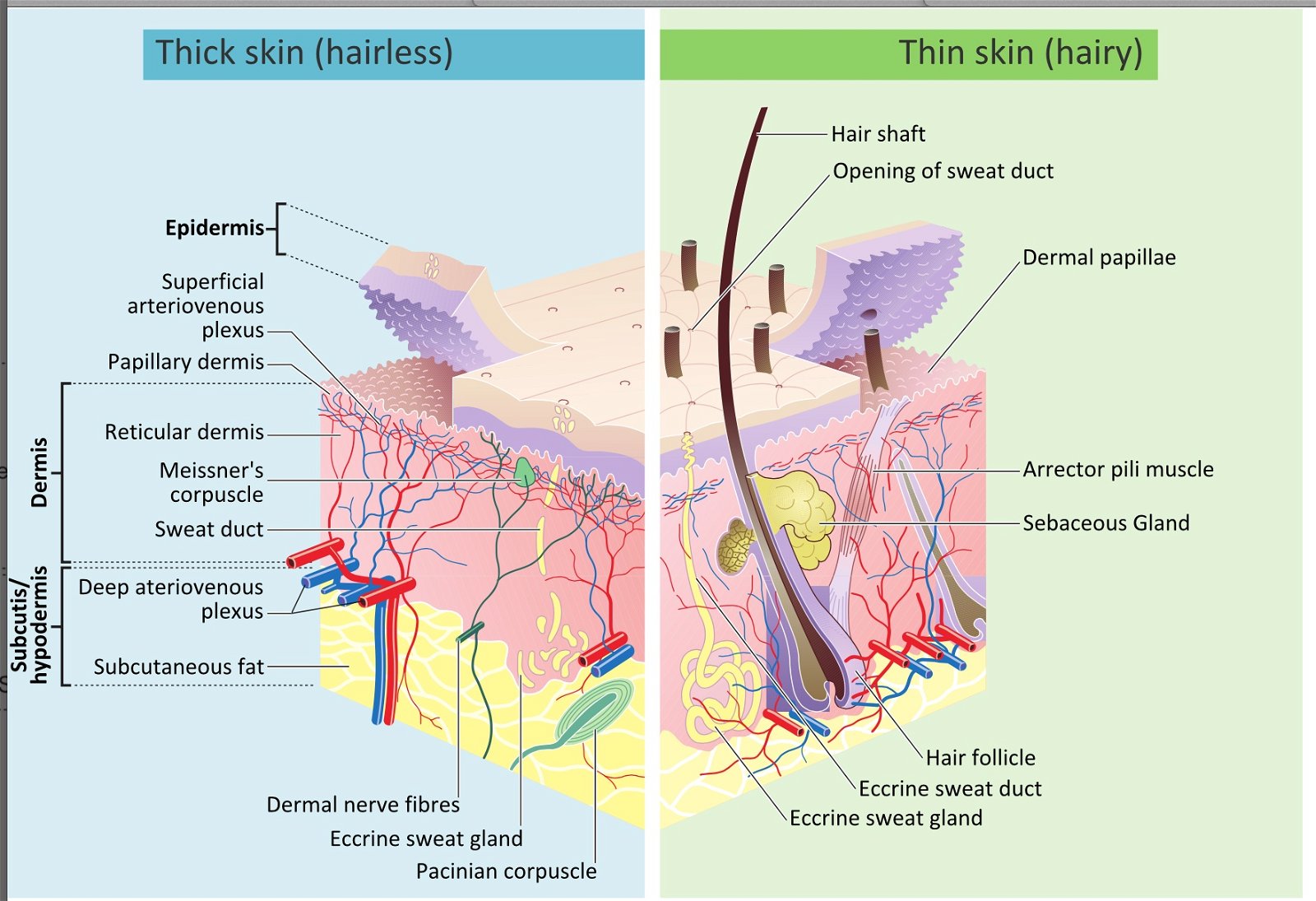 Fig. 9. The wondrous weaving the skin, Wikimedia commons.
Fig. 9. The wondrous weaving the skin, Wikimedia commons.
In the pre-Fall and perfect world, a beneficial human microbiota likely existed via a symbiosis with the human body that typically resulted in commensal and mutualistic relationships. This “very good” interweaving between the components of the human body systems and the various bacterial and fungal species present on and within the body served to help the human body function optimally. The skin and its microbiome still illustrate an awesome, interwoven complexity, intricacy, and design beyond description, even in this fallen world (Figs. 9 and 10).
Two thousand years after the writing of Psalm 139, Andreas Vesalius began to unlock the mysteries of the human body when he wrote De Humani Corporis Fabrica. Fabrica was an excellent title for this new anatomical encyclopedia (Gillen 2001). The word fabrica (or fabricae) is Latin for craft, trade, industry, workmanship, and the process of building, construction, and production. A related Latin word, fabre, is an adverb that means skillfully. We see parallels in the Scripture and in body design. In The Defender’s Bible, Henry Morris (1995) says in his annotations of Psalm 139:15, “curiously wrought” means embroidered, a striking description of the tissue that begins organizing, part by part, the beautiful structure of the whole infant. In Psalm Psalm 139:13, “hast covered” implies weave. In this Psalm David writes (under inspiration) about skin and body covering, that it was woven into our very being from the conception of life. Like the wondrous weaving of the skin into the blueprint of body’s covering (the skin and its microbiome), God’s plan for our life often appears like a woven tapestry.
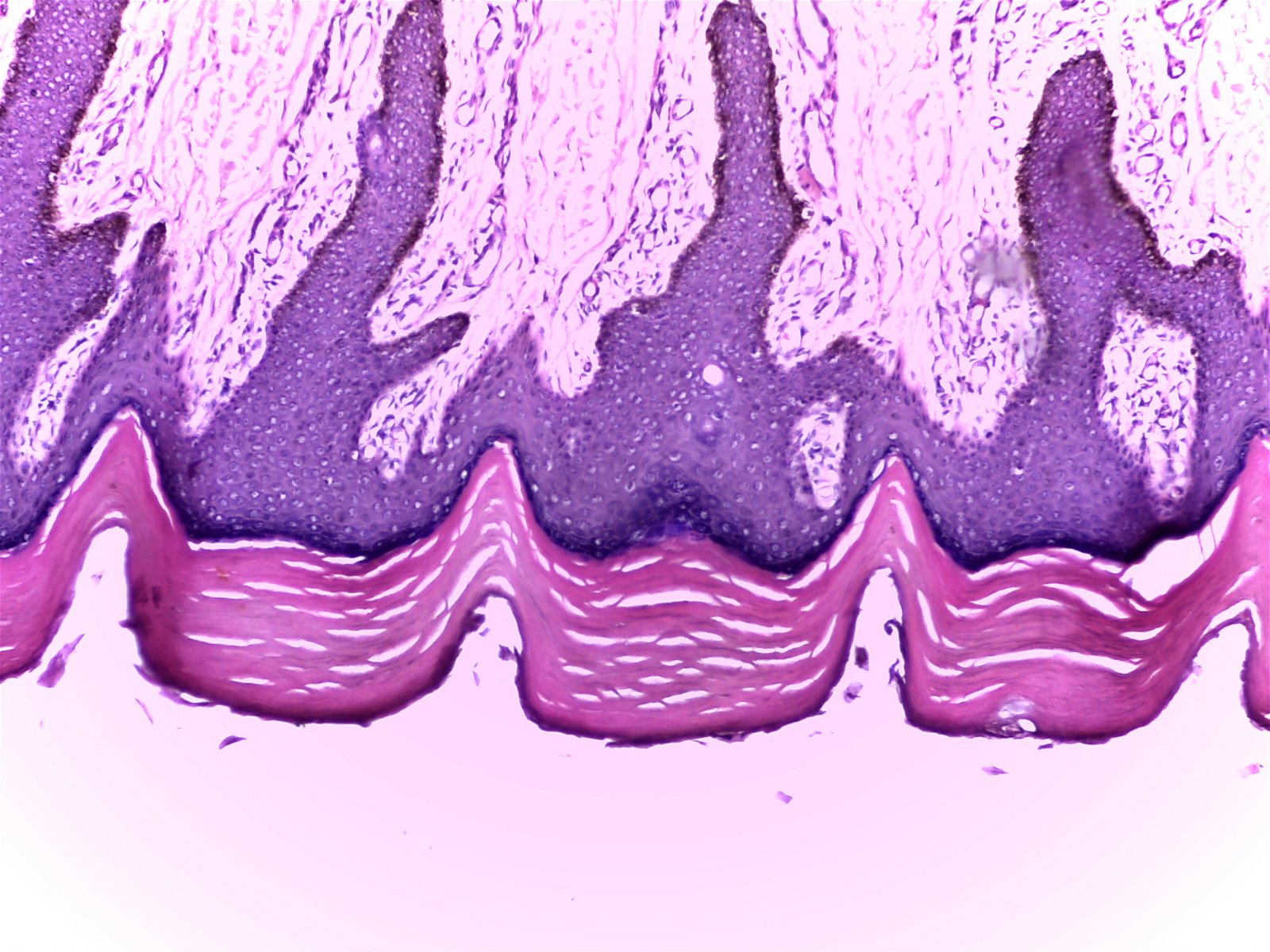 Fig. 10. Thick skin (100X), Alan L. Gillen image.
Fig. 10. Thick skin (100X), Alan L. Gillen image.
The fabric-of-life idea reveals that the Creator purposely wove together, piece by piece, the integumentary system (made up of four basic tissue types and microbiome). The Master Weaver also stitched a network of tissues containing dozens of specialized bacteria to form a finished masterpiece fabric that surpasses all others in intricacy, specificity, and beauty.
If skeptics would look closely at the human body, they would see all the individual “threads” (i.e., parts) seamlessly woven together (Figs. 9 and 10). Those viewing a rich tapestry with intricate patterns and vibrant colors would immediately praise the designer, because they realize that only a master designer could produce such a magnificent work of art. How can these very same people then claim that the infinitely more complex human body happened by chance? The body, sewn together more skillfully than any man-made tapestry, contains many multi-faceted, interrelated, and interdependent systems that are masterfully woven together by the Master Craftsman.
Postscript
What about the experiment with the new bacterial skin tonic, developed by AOBiome? The early outcomes of this experiment are positive; but the long-term effect is still not known. The use of a probiotic skin “tonic” is promising and does build on the idea that “good” bacteria were designed to interact and benefit our skin (just like probiotic products like yogurt benefits our digestive tract). But, I would not throw away your soap just yet.
References
Baron, S. 1996. Medical microbiology. Galveston, Texas: University of Texas Medical Branch at Galveston.
Bauman, R. 2014. Microbiology with diseases by body system, 3rd ed. San Francisco, California: Pearson Benjamin/Cummings Pub. Co.
Brand, P. and P. Yancey. 1980. Fearfully and Wonderfully Made. Grand Rapids, Michigan: Zondervan Publishing Co.
Caryl, J. 2012. Your microbiome and you (part II): Skin, mentalindigestion.net/2010/01/15/your-microbiome-and-you-part-ii-skin.
Francis, J. W. 2003. The organosubstrate of life: a creationist perspective of microbes and viruses, R. L. Ivey, ed. Proceedings of the Fifth International Conference on Creationism, pp. 434–444. Pittsburgh: Creation Science Fellowship.
Garrity, G. M., ed. 2005. Vol 2: The proteobacteria. Bergey’s manual of systematic bacteriology, 2nd ed. Berlin: Springer-Verlag.
Gillen, A. L. 2009a. Body by Design: Fearfully and Wonderfully Made, 6th printing. Green Forest, Arkansas: Master Books.
Gillen, A. L. 2009b. The Genesis of Methicillin-Resistant Staphylococcus aureus. Answers in Depth 4.
Gillen, A. L. 2007. The Genesis of Germs: Disease and the Coming Plagues in a Fallen World. Green Forest, Arkansas: Master Books.
Gillen, A. L. and J. Conrad. 2014. Our Impressive Immune System: More Than a Defense. Answers in Depth 9.
Morris, H. M. 1995. The Defender’s Study Bible. Grand Rapids, Michigan: World.
Naik, S., N. Bouladoux, C. Wilhelm, M. J. Molloy, R. Salcedo, W. Kastenmuller, C. Deming et. al. 2012. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 337, no. 6098: 1115–1119.
National Institutes of Health Human Microbiome Project. 2014. Microbiome analysis and reference genome projects. Retrieved from hmpdacc.org/catalog .
NIH/National Institute of Allergy and Infectious Diseases. 2012. Protective role of skin microbiota described. ScienceDaily, www.sciencedaily.com/releases/2012/07/120726153947.htm.
Park, B., T. Iwase, G. Y. Liu. 2011. Intranasal application of S. epidermidis prevents colonization by methicillin-resistant Staphylococcus aureus in mice. PLoS ONE 6, no. 10: e25880, doi: 10.1371/journal.pone.0025880.
Reid, A. and S. Greene. 2013. Human Microbiome FAQ. Washington, DC: ASM Press.
Roberts, L. S., J. Janovy, Jr., and S. Nadler. 2013. Schmidt and Roberts’ Foundations of Parasitology, 9th ed. Boston, Massachusetts: WCB McGraw-Hill.
Scott. 2014. My No-Soap, No-Shampoo, Bacteria-Rich Hygiene Experiment. NYTimes.com, May 24, 2014, www.nytimes.com/2014/05/25/magazine/my-no-soap-no-shampoo-bacteria-rich-hygiene-experiment.html.
Tortora, G. J., B. R. Funke, and C. L. Case. 2013. Microbiology, An Introduction, 11th ed. San Francisco, California: Pearson Benjamin/Cummings Pub. Co.
Wilson, M. 2004. Microbial inhabitants of humans: Their role in health and disease. Cambridge, UK: Cambridge University Press.
Footnotes
- Wove together—this implies a mutualistic relationship between two distinct organisms, not a mosaic of one.
- The term human microbiome is so new that there is not yet a fully agreed upon definition. But two definitions, one genetic and one ecological, are important, according to Reid and Greene (2013):
Definition 1: Just as the entire collection of human genes is called the human genome, this definition of microbiome means the entire collection of genes found in all of the microbes associated with a particular host.
Definition 2: Ecologists use the term “biome” to describe the collection of plants and animals that live in a particular environment. Thus there are various terrestrial and aquatic biomes that are characterized by similar climatic conditions and collections of organisms. When microbiome is used in this sense, it refers to the ecosystem made up of microbes within and on the human body—that is, the collection of microbes that live in the human “habitat,” (definitions modified from Reid and Greene, 2013).
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis