
How Did Plants Survive and Disperse after the Flood?
Abstract
Biblical creationists are often asked about plant dispersal and propagation after a worldwide, devastating Flood. How many plants and seeds were brought aboard the Ark by Noah? Did some plants and seeds survive the Flood by means of riding atop vegetative mats, or by simply floating along? If so, how were these survivors able to propagate or re-seed after the Flood? Could some plants have survived as airborne seeds or spores? Or were they carried to the different continents around the world by human or animal vectors? The purpose of this paper is to address these and other questions regarding post-Flood plant survival and dispersal, and consider mechanisms by which this may have occurred.
Introduction
Plants do a divinely miraculous thing with energy from the sun. They turn sunlight into food. This is essential for animals and mankind because they can't eat sunshine. While early in life animals (and humans who share many similarities) possess all the main structural body parts they will ever have, plants constantly produce new structures throughout their life. Living plants maintain embryonic tissues that both regenerate themselves and continuously generate the basic structures (leaves, roots, stems, flowers and fruits, or cones). However, the way these new growth and reproduction structures are produced may be affected by the environmental conditions the plant is trying to occupy.
Plants can control or regulate their internal functions. Like animals, plants produce chemicals called hormones, which are produced in one part of the plant to signal cells in another part to respond. Examples of this are when flowering plants bloom at the most favorable times, when fruit ripens, and when trees lose their leaves in the winter. This ability of plants to add, shrink, or dislodge parts (leaves, stems, flowers, fruits) as necessary to survive gives them a unique design. Unlike animals, plants cannot pick up their entire bodies to find food; however, plants are equipped with other mechanisms enabling them to respond to the environment.
The study of an organism’s response to ecological growing conditions is known as environmental physiology. Stress from water loss, air chemistry, crowding by other plants, and flooding can change the way a plant functions. These variations may be affected by genetic, chemical, and/or physical factors.1 Given the intense topic of climate change we are seeing in today’s modern world, this is an especially popular area of study.
Seed Types and Dispersal Mechanisms
Plants have the ability to use and establish new lands for resources by various seed dispersal and rapid colonization traits. When a mature seed is in unfavorable conditions, it can undergo dormancy (a resting state) until surroundings are right. The particular structure of a plant species’ body, fruit, and seed dictate the means of dispersal. Some of these adaptations include the following: nutritious fruits to attract wildlife, buoyant thick-shelled nuts that float thousands of miles, dust-like seeds produced in the millions, winged or plumed seeds, and explosive fruits that can toss their seeds several feet. Seeds can be packaged in cones (pine trees), pods (honey locust), capsules (willow), nuts (chestnut, oak), with wings (ash, elm, maple) or with varying fleshiness of fruit coverings (raspberry, cherry, apple).2 The Hawaiian flora and fauna are representative of long distance dispersal for plants. The plant colonizers that survived the long journey across the Pacific had seeds that were tolerant to salt water or small enough to be carried in the wind or by birds.3
Seeds can cross oceans via birds (digestive tract or attachment), by wind (air currents), or by waves (rafting in ocean currents). Seeds of the Australian pine (Casuarina) survive immersion in salt water indefinitely but are not buoyant. These plant seeds are believed to cross oceans by rafting, particularly on floating volcanic pumice upon which they have been seen germinating.4
This protective outer layer helps protect the internal plant embryo from injury or from drying out. Seed coats are important in the longevity of the seed. Seed longevity is an ecological characteristic of a plant as well as a physical and a chemical one. The growth form of plant species, their type of seed dispersal, is adapted to the habitat in which they are commonly found. A thin seed coat provides no barrier to water, but allows light to quickly penetrate, triggering the end of seed dormancy.5 Some plants are primary pioneers. They ordinarily grow on tough sites where soil is scarce or poor. How do plants revegetate a burned area as promptly and abundantly as they do? Simply because they have programmed, durable, heat-resistant, and long-lived seeds. Intense heat from a fire can break seed dormancy in some plants (Acacia). The chemical barrier on their seed coats is disrupted, thereby triggering extensive seed germination.6
When Herod the Great's palace was excavated in Israel (1963), researchers discovered date palm seeds preserved in an ancient jar. The University of Zurich confirmed the seeds dated from between 155 BC to AD 64. After an additional 40 years, the seeds were pretreated in fertilizers and a hormone-rich solution, and then planted (2005). What grew is one of the oldest known tree seeds successfully germinated, and the only living Judean date palm, a tree thought extinct for over 1,800 years. The plant is called “Methuselah,” named for the oldest person recorded in the Bible.7,8 Ancient hazelnut-sized Manchurian seeds were found in a peat layer in a dry lake bed in China. The seeds have very thick protective seedcoats. Several germination tests were done, and most all of the seeds grew. On several seeds, age tests suggested they were between 830 and 1,250 years old.9
Tropical drift seedpods and fruit nuts are extraordinary because they can survive months or even years at sea. They are very buoyant with thick protective shells that are impenetrable to salt water. In some drift fruits, such as the coconut, the seed embryo and fleshy white “meat” (endosperm) is enclosed within a hard layer (endocarp) surrounded by a thick husk. Other drift seeds have thick woody coats and internal air cavities that make them buoyant. During their long voyages, these seeds often cross entire oceans (table 1).10 Because many animals died in the Flood, their carcasses could have floated on the surface of the waters, holding and protecting seeds in their bodies. Early experiments by Darwin found that many kinds of seeds in the crops of floating birds can retain their ability to germinate up to 30 days.11
Did you know fish can act as a mechanism for seed dispersal? Cattle, sheep, horses, deer, bear, rabbits, birds, and fish are also known to pass viable seeds. The technical term for this is endozoochory. During the Flood of Noah’s day, freshwater and marine fish could have survived in water suited to them, in spite of being temporarily displaced from their normal habitats. The gamitana fish (Colossoma macropomum), of Peru, eats mostly fruit and can transport seeds down the Amazon River up to three miles. Researchers examined 230 fish and found nearly 700,000 intact seeds from 22 plant species, representing 21 percent of the species that fruit during the flood season. The relationship between these fish and plants is based on the seasonal rains, which can flood areas for up to nine months with water 19 feet deep for nearly five months. During the rainy season, these fish spend 90 percent of their time in the flooded habitats, waiting for fruit to fall into the water12
| Beach bean (Canavalia maritima) | Asian swamp lily (likely Crinum asiaticum) |
| Coin plant (likely Dalbergia monetaria) | Dog almond (Andira inermis) |
| Hog plum (Spondias mombin) | Grenade pod (Sacoglottis amazonica) |
| Mammee apple (Mammea americana) | Beach morning-glory (Ipomoea pes-caprae) |
| Manchineel tree (Hippomane mancinella) | Yellow nickernut (Caesalpinia ciliata or C.major) |
| Mango (Mangifera indica) | Oak acorn (Quercus sp.) |
| Nothing nut (Cassine xylocarpa) | Sea bean (Mucuna urens) |
| Sandbox tree (Hura crepitans) | Pod (possibly Sterculia sp.) |
| Sea coconut (Manicaria saccifera) | Sea heart (Entada gigas) |
| Seaside hibiscus (Thespesia populnea) | Gray nickernut (Caesalpinia bonduc) |
| Sugar apple (Annona squamosa) | Red mangrove (Rhizophora mangle) |
| Tamanu (Calophyllum inophyllum) | Coconut endocarp (Cocos nucifera) |
| Tropical almond (Terminalia catappa) | Calabash (Crescentia cujete) |
| West Indian locust (Hymenaea courbaril) | Box fruit (Barringtonia asiatica) |
There are two methods of plant reproduction: sexual (seed) and asexual (vegetative). Seed production by flowers or cones requires the transfer of pollen: a sharing of genetic material between two plants. In nature this results in offspring that differ from each other and from their parents. Vegetative propagation is designated “clonal” by scientists: young progeny are genetic copies of the parent plant.
Seed germination requires oxygen and water. Following pollination, the development of viable seeds may or may not occur; a great deal depends upon environmental conditions. A severe freeze, snow, or rain event at the time of blooming can eliminate the seed cycle for that year. Even if viable seeds are produced and expelled, they may be forced to wait to germinate until some later year when conditions are more favorable. The most reasonable view, widely held by plant experts, is that seed dormancy is not only associated with the absence of germination, but it is also a seed characteristic that determines the conditions required for germination.13
Many plants have alternative methods of reproduction, the most common being through vegetative rhizomes. Rhizomes are creeping, underground, root-like stems that run out from a plant with the ability to send up a new shoot, i.e., new clone-like plant. A single rhizome plant can occupy an area of several feet with its roots growing in an interconnecting system. This plant feature is an adaptation to fill an area rapidly.
Colonization in Extreme Conditions
As plants are a major component of food production in our world, seed crop research is a major emphasis in the 21st century. Since humans cannot create more land, researchers are investigating how to rapidly increase plant growth and seed production. Arabidopsis thaliana grows in many areas throughout the world and was the first plant to have a completed genome sequence. The sequence showed a very simple plant whose entire genome consists of a relatively small set of genes that dictate when the weed will bud, bloom, sleep, or seed. This plant is a member of the cabbage family known as Cruciferae due to its uniform flower structure that resembles a cross. A. thaliana is self-pollinating, with a rapid life cycle (5–7 weeks). This abundant seed producer is a worldwide celebrity genome plant because of its physical capabilities and relatively simple genetic traits. Only a handful of genes are known to be directly involved in determining its seed size.14 So when A. thaliana plant researchers claim “evolution might play a role in how fast a species can move across a region or continent,”15 it is already evident that plants can/do adapt and grow rapidly into barren, new, isolated, or hostile growing areas, as the Creator gave them provision to do in a cursed world. When laboratory experiments don’t allow new traits to enter the A. thaliana plant population, they claim they have “stopped evolution.”16 In reality the plant is continuing to grow at a more constant rate because it is not being influenced by any environmental change. In nature there is always change: wind, rain, light, temperature fluctuations, floods, droughts, herbivory (browsing by animals), man-made influences, and so on. Further plant adaptability examples will establish how plants are affected by environmental change—note that this is not molecules-to-man evolution, as suggested by evolutionary research in plant migration. When extreme changes occur, plants can be kick-started to rapid growth or to a steady state of reserve (dormancy).
Drought or lack of available water
Plants can harvest low concentrations of nutrients if the soil moisture content is limited. Scientists conducted a study of water transfer between interconnected vegetative growing roots of mother and daughter plants (Carex grass). When the interconnected pair was exposed to an uneven water supply, acquired water was transferred (30–60%) from the wet plant to the dry plant.17
High Salinity
It is not likely the salinity was the same during the inundation stages of the Flood as it is now. But even if it were, plants would have been able to recolonize.18 Habitat selection of plant species with rhizomatous growth was documented in a study of western ragweed (Ambrosia psilostachya). The selective placement of vegetative roots into nutrient-rich or optimal sites may enable these plants to actively choose habitats for future growth. Opportunities for habitat selection in natural populations depend on the rate of rhizome growth into new territory. In one field experiment, rhizome growth in nonsaline soil was small (3%) and at a very short distance from their parent plants. However, dispersal in saline soil was dramatically higher, with almost one-third of all daughter plants appearing farther from their parents. What was happening? These plants did not prefer saline soil, so they grew faster and farther to get away/out of it. The growth commitment to the extensive rhizome root system increased the rate at which plants in saline soil could encounter new territory.19 In many salt marshes, plant species can survive indefinitely with only minute migrations. Many salt marsh plants are known to be capable of long-range dispersal by ocean currents, such as saltwort (Salicornia spp.), which has seeds capable of floating for months.20
Flooding
The vast diversity of seed dormancy mechanisms and treatments to overcome dormancy complicate a universal consensus on the definition of the word dormancy. A unique grass species (Orcuttia pilosa) starts life as a submerged aquatic seedling, develops floating leaves, becomes a tall, emergent grass when the water level drops, and then flowers within days. The grass seed germinates only when infested with certain fungi, which are abundant only when the pond is flooded. In years when their home pond has not flooded, the plant seeds have remained in the pond soil for up to four years, then germinated with rain. Water hyacinth (Eichhornia crassipes) also stores dormant seed in the mud, where it retains its viability for 15 years or longer.21
Flooding fills the gas exchange pores on seeds and limits oxygen transport. Depending upon the species and duration of flooding, seed growth ability varies. Some seed studies have seen plants emerge after four years (Schoenus nigricans, Carex appropinquata and C. davalliana).22 For example, bald cypress, water tupelo, and black tupelo seeds remain viable for extended periods of flooding. These seeds wait for floodwater to recede, then germinate. To quickly establish new areas created by flooding, cottonwood, willow, and sycamore seeds can germinate submerged in water.23
Freezing temperatures
Most alpine tundra plants likewise reproduce through rhizomes; moreover their roots are 2–6 times longer and wider underground than the plant height above ground (Figure 1). Alpine plants can carry on rapid rates of metabolism when the ground begins to thaw. These plants rely heavily on food reserves stored in old evergreen leaves (another adaptation) and in large root systems underground.24 This enables the plants to rebound vigorously when spring arrives.
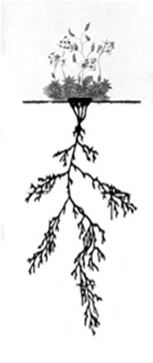
Figure 1. Alpine Draba plant with roots three times the bulk below ground. Image adapted from Atlas der Alpenflora.
Extreme Conditions and Effects on Plant Community Succession
When stripped of its original vegetation by fire, flood, or glaciers, areas of bare ground do not remain devoid of plants and animals for very long. The area is rapidly colonized by many types of plants. This newly colonized environment allows wildlife species and new plant species to become established, which in turn allow even other plants, mostly trees, to grow. The transitional series of plant communities that develop are called stages, while the final stable and mature habitat is called a climax community. Ecological succession is the term for the community’s development by action of vegetation leading to the establishment of new inhabitants. Succession is the universal earth process of directional change in vegetation during time. It can be recognized by the progressive change in the species composition of the community being observed.
The mechanism of vegetation succession (Figure 2) is foundational for wetland restoration and conservation, and has become a central issue of wetland science research. Although several factors (such as soil nutrients, moisture levels, and plant competition) influence the succession of wetland vegetation, water (hydrological) conditions are the most significant and complicated elements. Wetland hydrology includes water-level fluctuation, flood inundation probability, hydro-period related variables (start time, duration, and end time of flooding), and sedimentary material. The wetland landscape pattern can present habitat patches for adapting to hydrological processes at different levels. Changes in wetland landscape patterns can provide a comparative analysis of how wetland plants adapt and respond to flooding stress.
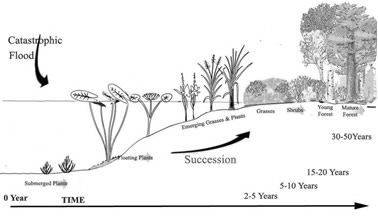
Figure 2. Plant succession (growth stages) from a ‘wet’ land to a mixed forest. Image adapted from U.S. Geological Survey by G. Allen.
Recent studies in China (1993–2010) and the United States (Mount St. Helens 1980–2015) show that recovery of plant and animal systems is faster than many people predicted, that this recovery progresses in stages, but also that adaptation to the environment is intrinsic: it is part of the primary nature of plants. In the Mount St. Helen study, much of the plant recovery was a trial-and-error process, with seeds blown in on the wind and animals traveling to patches of surviving plants. One kind of plant that thrived after the eruption—and assisted the landscape to be more suitable for other plants—were the lupins. These flowered legumes were some of the only plants that could grow on the pumice around the volcano. The volcanic rock is low in some essential nutrients, and is not conducive to most kinds of plants; lupins, however, can make these nutrients themselves, enabling them to grow and gradually add nutrients to the soil for other plants to grow. The initial devastation determined what could grow and what couldn’t, but the area gradually returned to a pre-disaster, mature-plant community with most of the previous plant species present in a short 30 years.25
In China, floods played a critical role in accelerating the process of wetland sedimentation, which in turn had significant influence on wetland vegetation succession. Fine sediments transported by floodwaters can provide accumulation of soil nutrients.
Furthermore, the deposition of these sediments in wetlands generally raises the elevation (slope) and decreases new flooding effects. Less than one percent (0.6%) of wetlands in this study were negatively influenced by flood duration. Extents of reed and meadow wetlands expanded and were gradually moving forward in a matter of less than two decades. The speed of sediment deposition generally determined the vegetation succession process.26
Making Themselves at Home
Environmental change is any change in an environment to which a plant must respond by species adaptation or individual physiological flexibility. Change can be gradual, such as from mountains or deserts forming, or a change can be quick, such as from floods, volcanoes, or earthquakes.
Discussion in Regards to the Noachian Flood
We see from Scripture that the first indication of plant life returning after the global Flood is the olive leaf that was brought back by the dove (Genesis 8:10–11). So instead of seeds or plants needing to survive for an entire year in or under water-soaked soil, they would have only had to endure water for a maximum of just over nine months, and those that had hitched a ride on large mats of vegetation or on carcasses could be germinating while protected from the harsh conditions.27
We don’t know how much mixing of fresh and salt waters would have occurred during the Flood or exactly what the salinity of the water was post-Flood.28 What we do know is, due to abundant dormant buds in the wood, olive is easy to propagate and was widely cultivated in ancient times.29 Olive trees are very tough and resistant to drought, disease, and fire. Moderately salt tolerant, these trees can live for hundreds of years. The olive tree root system is very vigorous and capable of regenerating the tree even if the above ground structure is destroyed. Branching side shoots or underground runners (suckers) sprout readily from olive stumps or broken branches. Since olive trees are this hardy, the concept of a floating olive tree sprig surviving and sprouting in a short period of time is completely reasonable, even after the catastrophic Flood.

Figure 3. Modern day depiction of a dead floating log with growing plants. Image by Superbass, via Wikimedia Commons.
The Ark Encounter in Williamstown, Kentucky, depicts active effort by Noah and his family to preserve some types of plants (particularly food sources) while aboard the Ark.30 Noah would likely have preserved as many plants as he could. Noah may have preserved seeds and cultivated plants so that there would be fresh food to eat during the one-year voyage. Noah may have potted seedlings to preserve useful trees and shrubs. The Ark’s upper deck beneath the long window may have been specially designed to accommodate these plants, turning part of the Ark into a vast greenhouse.
Most plants could have survived outside the Ark upon floating rafts of vegetation as seeds and as debris that could have gone a long way toward propagation of at least some plant life in the post-Flood world.31 And this brief exercise demonstrates that it is conceivable that the rest of the post-Flood plants were recolonized both from seeds, which remained dormant during the Flood, and from vegetative propagation of one form or another.
Plants are extremely well adapted to specific growing elements, and since reproduction is a survival necessity, plants were equipped “from the beginning” with a variety of reproductive mechanisms. The precise, timely, and hardy responses to environmental changes by various plant-growing traits exemplify God’s awesome benevolence and intelligence in designing plants and enabling their survival even in a cursed world. As we have seen, following many climate or irregular earth process catastrophes, God's creation was given an amazing ability to reestablish itself, and natural system recovery always begins with the appearance of plants.
Footnotes
- Anthony J. F. Griffiths et al., “Genes, the Environment and the Organism,” chap. 1 in An Introduction to Genetic Analysis, 7th edition (W.H. Freeman, New York, 2000): https://www.ncbi.nlm.gov/books/NBK21842.
- R.O. Parker, Introduction to Plant Science (Abingdon: Taylor & Francis, 2004), 277.
- Carolyn Corn, “Hawaii Seed Dispersal Methods In Hawaiian Metrosideros,” (Honolulu, Hawaii: Island Ecosystems IRP, U.S. International Biological Program, 1972), https://scholarspace.manoa.hawaii.edu/bitstream/10125/15266/1/06.pdf.
- Jonathan D. Sauer, Plant Migration: The Dynamics of Geographic Patterning in Seed Plant Species (Berkeley, CA: University of California Press, 1988), http://ark.cdlib.org/ark:/13030/ft196n99v8/.
- Rick Parker, Plant & Soil Science: Fundamentals & Applications (Boston, MA: Cengage Learning, 2009), 333.
- Brian James Atwell, Plants in Action: Adaptation in Nature, Performance in Cultivation (London: Macmillan Education AU, 1999), 596.
- “Methuselah,” Arava Institute, http://arava.org/arava-research-centers/arava-center-for-sustainable-agriculture/methuselah/.
- Clara Moskowitz, “Extinct Tree from Christ’s Time Rises from the Dead,” Live Science, June 12, 2008, http://www.livescience.com/2602-extinct-tree-christ-time-rises-dead.html.
- Clarence R. Quick, “How Long Can a Seed Remain Alive?,” in The Yearbook Of Agriculture (Washington, D.C.: The United States Department of Agriculture, 1961), https://naldc.nal.usda.gov/download/IND50000162/PDF.
- W. P. Armstrong, “Ocean Drift Seeds and Fruits,” Wayne’s Word, December 3, 1998, http://waynesword.palomar.edu/worthypl.htm.
- David Wright, “How Did Plants Survive the Flood?,” Answers in Depth 7 (2012): October 2012, https://answersingenesis.org/the-flood/how-did-plants-survive-the-flood/.
- Krishna Ramanujan, “Overharvested Amazon Fish Disperse Seeds Long Distances,” Cornell Chronicle, April 18, 2011, http://www.news.cornell.edu/stories/2011/04/overfished-amazon-fish-disperse-seeds-long-distances.
- William E. Finch-Savage and Gerhard Leubner-Metzger, “Tansley Review: Seed Dormancy and the Control of Germination,” New Phytologist 171 (2006): 501–523, http://www.seedbiology.de/html2/dormancy06-abs.html.
- “First-Ever Complete Plant Genome Sequence Is Announced International Team Reveals DNA Secrets of Arabidopsis thaliana,” National Science Foundation, December 13, 2000, https://www.nsf.gov/od/lpa/news/press/00/pr0094.htm.
- University of British Columbia, “Evolution Drives How Fast Plants Could Migrate with Climate Change,” ScienceDaily, July 28, 2016, http://www.sciencedaily.com/releases/2016/07/160728155005.htm.
- Ibid.
- Hans de Kroon et al., “High Levels of Inter-Ramet Water Translocation in Two Rhizomatous Carex Species, As Quantified by Deuterium Labelling,” Oecologia 106, no. 1 (1996): 73–84.
- David Wright, “How Did Plants Survive the Flood?”
- Amy G. Salzman, “Habitat Selection in a Clonal Plant,” Science 228, no. 4699 (May 1985): 603–604, doi:10.1126/science.3983647.
- D. S. Ranwell, Ecology of Salt Marshes and Sand Dunes (London: Chapman & Hall, 1972), 258.
- Jonathan D. Sauer, Plant Migration: The Dynamics of Geographic Patterning in Seed Plant Species (Berkeley, CA: University of California Press, 1988), 44.
- S. Tatár, “Seed Longevity and Germination Characteristics of Six Fen Plant Species,” Acta Biologica Hungarica 61 (2010): 197–205, doi:10.1556/ABiol.61.2010.Suppl.19.
- Kim D. Coder, “Flood Damage to Trees, College of Agricultural & Environmental Sciences,” University of Georgia, http://www.caes.uga.edu/topics/disasters/flood/articles/treedamage.html.
- Larry W. Price, Mountains and Man: A Study of Process and Environment, (Berkeley, CA: University of California Press, 1981), 296–298.
- Andrea Thompson, “Mount St. Helens Still Recovering 30 Years Later,” Live Science, May 17, 2010, http://www.livescience.com/6450-mount-st-helens-recovering-30-years.html.
- Yanxia Hu et al.,“Monitoring Spatial and Temporal Dynamics of Flood Regimes and Their Relation to Wetland Landscape Patterns in Dongting Lake from MODIS Time-Series Imagery,” Remote Sensing 7, no. 6 (2015): 7494–7520, https://www.researchgate.net/publication/279224526.
- David Wright, “How Did Plants Survive the Flood?”
- Andrew A. Snelling, “How Could Fish Survive the Genesis Flood?,” chapter 20 in The New Answers Book 3, ed. Ken Ham (Green Forest, AR: Master Books, 2010), 195–204.
- D. Zohary and P. Spiegel-Roy, “Beginnings of Fruit Growing in the Old World,” Science 187 (1975): 319–327 in Mohamed Chliyeh et. al., “Bibliographic Inventory of the Olive Tree (Olea europaea L.) Fungal Diseases in the World,” International Journal of Pure & Applied Bioscience 2, no. 3 (2014): 46–79.
- “How Did We Make the Lettuce and Cabbage Garden?,” Ark Encounter, July 21, 2016, https://arkencounter.com/blog/2016/07/21/how-did-we-make-lettuce-and-cabbage-garden/.
- “Noah’s Floating Farm of Animals and Plants,” Ark Encounter, August 31, 2012, https://arkencounter.com/blog/2012/08/31/noahs-floating-farm-of-animals-and-plants/.

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis

