Why New Diseases Keep Popping Up
A creation perspective on why God allows emerging and reemerging diseases like COVID-19 and Lyme Disease that cause major emotional, financial, and health problems.
Abstract
New and reemerging diseases are terrifying and concerning problems. Not only do they carry a financial and emotional toll, but they account for significant numbers of death. Emerging diseases are usually zoonotic and highly virulent in nature. These are pathogenic and parasitic diseases of high consequence and impact. Why would a good Creator allow these new diseases? Why do new diseases keep popping up? Mutation and displacement of original types of microbes account for many new diseases; however, the answer is more complex than just these two factors. Zoonoses are the most common type of infection, specifically from viral pathogens, bacteria, and parasites. Some of the more notable recent emerging diseases are COVID-19, Lyme Disease, and Giardiasis. An example of a disease that is not zoonotic is smallpox. In some locations, more malaria infections are “jumping” from monkeys to man. The displacement of microbes from their originally designed place in animals and the transfer to humans explain many of the world’s pandemics, plagues, and pestilences. Some goals of this article are to explain the concepts of displacement, mutation, and “One Health” while solving the riddle of pandemics, plagues, and pestilences. We will examine the relationship between humans, microbes, and animals and explore the principles of new and reemerging diseases by giving a few examples.
Introduction
COVID-19 has taken the world by storm and has dominated the headlines since early 2020. Most likely, the two greatest “plagues” (pandemics) of all time have been the Spanish Flu (1918–1920) and COVID-19. No other “plagues” have affected such high numbers (~180 million worldwide, John Hopkins 2021). Both diseases appear to have a zoonotic (animal) connection. In India, Asia, and much of the world, additional outbreaks of the dreaded COVID-19 have stretched into months.
COVID-19 is an example of a probable zoonosis (i.e., spillover, displacement) from bat (fig. 1) to human (Gillen and Conrad 2020). Some suggest the virus may have come from a biological lab leak in China and was being selected as a possible biological weapon. However, even if COVID-19 had an accidental lab origin, the original viruses they were cultivating still had their origin in wild bats. Bats are not affected by the SARS-CoV-2 virus (fig. 2) due to their amazing immune system; however, people have been greatly affected by this novel virus that leads to COVID-19. It joins the “parade” of other frightening new maladies that continue, each one confirming that humans, for all their cleverness, still live “at the mercy” of microbes and parasites. In fact, about 87 new or reemerging diseases (Chess 2021) have occurred since the 1980s, with one of the major contributors being urban sprawl into previous wilderness, jungle, or desert environments, which allows the viruses, pathogens, and parasites to become displaced. With this displacement, humans are exposed to animals carrying these new “germs,” and new strains of disease are born.
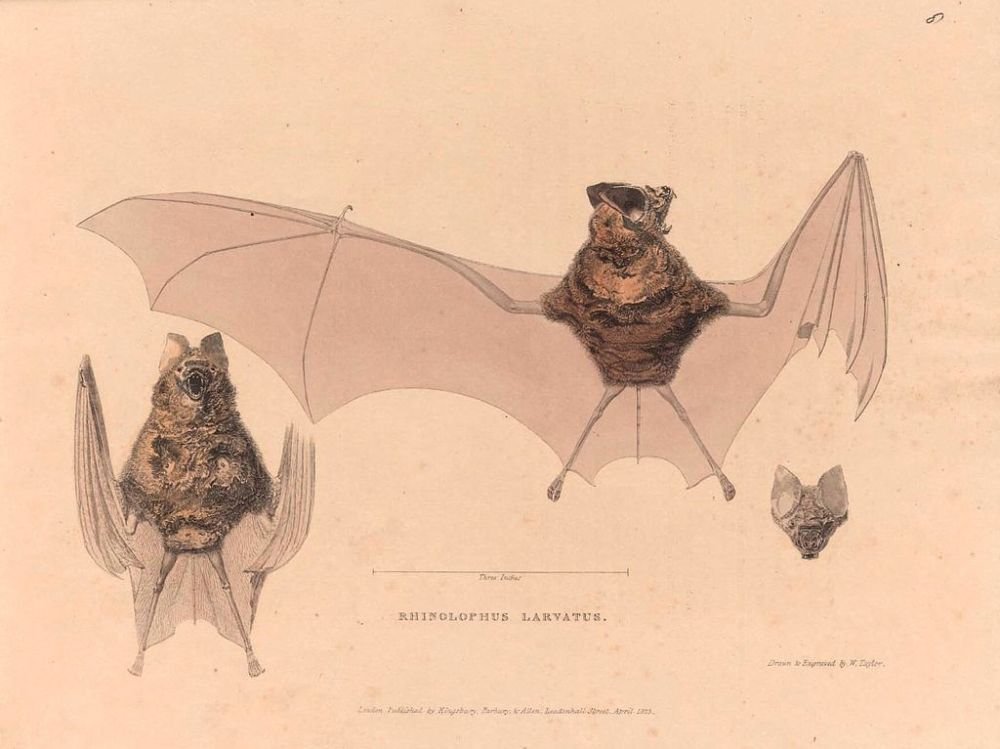
Figure 1. Three views of the bat Rhinolophus larvatus (Horsfield’s Leaf-Nosed Bat). Image by John Curtis, William Daniell, Thomas Horsfield, Charles Joseph Hullmandel, A. Pelletier, and W. Taylor via Wikimedia.
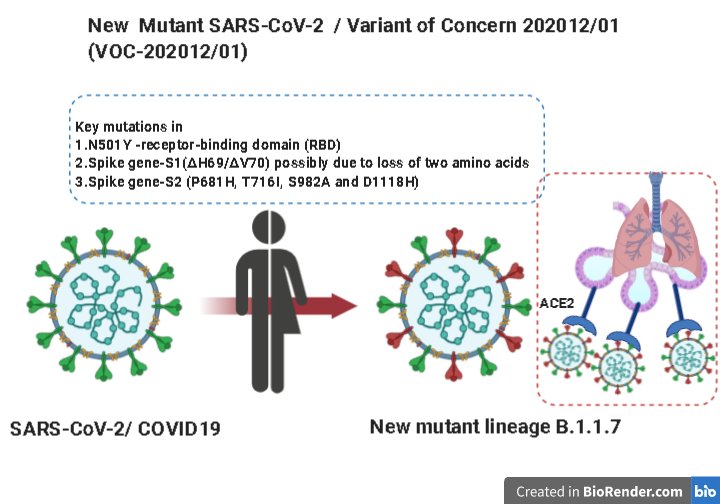
Figure 2. New variations of SARS-CoV-2 have emerged due to 3 key mutations. Image by Chinmayamahapatra via Wikimedia.
In fact, about 87 new or reemerging diseases (Chess 2021) have occurred since the 1980s, with one of the major contributors to new and emerging diseases being urban sprawl into previous wilderness, jungle, or desert environments.
Many of these pathogens’ origins are mysterious, but we do know that 75% of emerging infectious diseases have an animal connection. They are either zoonotic (animal infection) and/or vector-borne diseases that have an insect or tick carriage. There is danger as jungle (sylvatic) strains encounter urban strains and mix. As pointed out in The Genesis of Germs (Gillen 2020), the origin of infectious disease is complex and multifaceted. From a biblical worldview, infectious diseases and pathogenesis are a secondary condition, a result of sin, that was not what the Creator originally intended. The sequence of events in a biblical worldview might be seen as Creation, Curse, Corruption, Contagion, and Crisis.
Table 1. Fast Facts About Emerging Diseases
- There have been 87 totally novel new or reemerging diseases novel since 1980.
- The World Health Organization (WHO) tracks over 100 emerging and reemerging diseases.
- Sixty percent (or more) of known infectious diseases in people can be spread from animals.
- Three of every four new or emerging infectious diseases in people come from animals.
- Most common pathogens of high consequence include SARS-CoV-2, HIV infections, SARS-CoV-1, MERS CoV, Borrelia, Escherichia coli O157:H7 (E. coli), Yersinia pestis, hantavirus, dengue virus, West Nile virus, and the Zika virus.
“Why are there new diseases?” Medical scientists are asking and trying to answer these critical questions:
- What diseases are truly new?
- Why do new diseases keep popping up?
- What is the origin of these new, emerging diseases?
- How do secular biologists differ from those with a biblical worldview in explaining infectious diseases?
The Concept of “One Health”
Human, animal, and environmental health are intricately interwoven, inextricably interrelated, and providentially designed. All life on earth is connected by the Creator’s plan. Secular scientists (including those at the CDC) recognize the connection within the biosphere and call it One Health (Cowan and Smith 2021). The idea is that all three interacting components are connected and that good stewardship recognizes the importance of all. The reasoning is that microorganisms (and parasites) circulate among human hosts, animal hosts, and environmental reservoirs. Changes in the environment can lead to the new transmission of pathogens to animals and humans. It might be helpful to visualize one health (fig. 3) as three overlapping spheres. A change in in any of one of the spheres influences the others as it happens continuously. The mixing of microbes in different animal hosts (displacement) and under different environmental conditions can lead to the adaptation of potentially new pathogens and parasites.
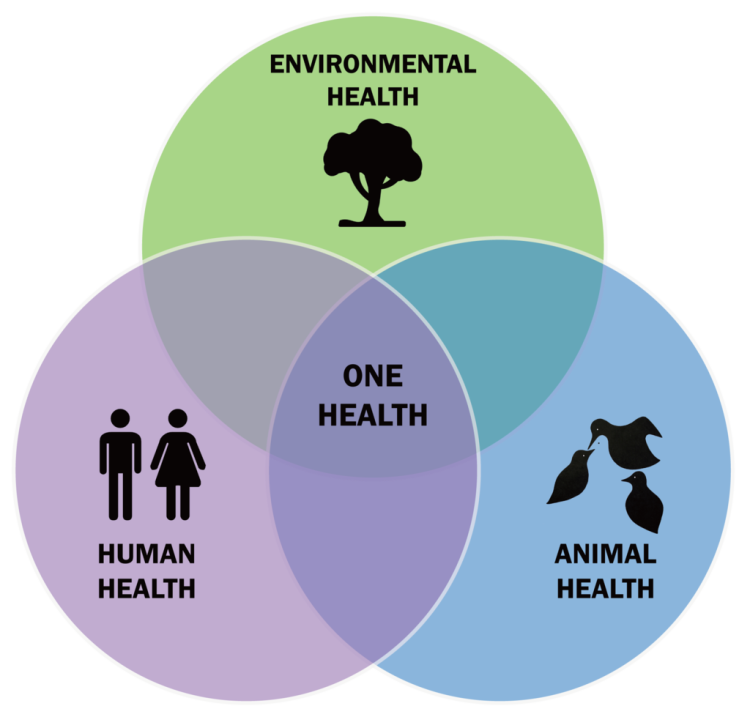
Figure 3. The one health concept consisting of a triad of human, animal, and environmental health’s. Image by Thddbfk via Wikimedia.
Human activities can promote the emergence of infectious diseases (e.g., through ecological disturbances, disruption of wild-animal habitat, eating exotic animals, and the movement of animals (including livestock). This has occurred recently with swine flu and COVID-19. Interactions of birds, swine, and humans resulted in the change of influenza recombinant strains. Humans’ eating exotic animals may have resulted in SARS.
Changes in the environment, such as warmer weather, heavy rain, and drought can induce adaptive mutations. Changes in temperature can alter the habitats of disease-carrying arthropods (fleas, ticks, mosquitoes) and lead to changes in who is at risk. The Zika and Dengue viruses move north due to their host Aedes aegyptii edging ever northward as the temperatures increase even slightly there. Sometimes changes in environment or in the types of animals that humans frequently encounter can lead to gradual or sudden changes in host specificity of microbes. The sum of this interconnectedness is the acceleration of newly emerging disease as well as the reemergence of old diseases (in new forms) once thought to be under control. WHO has a list of 100+ diseases that they track and consider the greatest threats. There have been 70 totally new (novel) emerging diseases since 1980 (Chess 2021).
Climate Change Affecting Emerging Diseases Emergence
While there is evidence that changing weather patterns (rain vs. drought) and temperature fluctuations are affecting new and reemerging diseases, evidence of global warming from carbon output is somewhat in question. We have witnessed in our own research how the presence of beavers and rainfall increases the concentration of Giardia and its spread downstream. This phenomenon occurs due to the escalation and resuspension of particulate matter from the bottom of the water column following surface runoff (Gillen 2021). Beavers increased in abundance after prolonged rainfall for a year, and so did Giardia. Although the Giardia did not seem to cause disease in the beavers, it appears to have spread to humans and domestic animals, resulting in increasing cases of Giardiasis in dogs and cats in Virginia. This observed change locally does correspond to the concept of “One Health.” There is an interconnection between animals, humans, and the environment. However, controlling global “climate change” and whole planet factors is probably beyond human control.
Displacement Analogy
Displacement of microbes/parasites to new ecological niches due to genetic alterations or changes in the host or environment allowing symbiont to explore new ecological niches (with or without genetic alteration) may also play a role in pathogenicity/parasitism. Though originally created good, the curse of Genesis 3 allows them to become pathogenic when they’re in a new position or place. A scalpel in the hand of a skilled surgeon is useful, but it can cause havoc in the hands of a murderer. Pathogens and parasites are harmful when they show up where they should not be. This is referred to as displacement theory in creation biology. Some of the most terrifying and concerning diseases are zoonotic diseases. Most zoonotic diseases can be explained by the displacement and/or mutation of the original kinds. These are the diseases that are highly virulent with high consequence and impact.
Spillover Zoonoses
Most spillover events result in self-limited cases with no further human-to-human transmission, as occurs, for example, with rabies and anthrax (fig. 4). Other zoonotic pathogens can be transmitted by humans to produce secondary cases and even to establish limited chains of transmission. Some examples are the Ebola filoviruses, SARS-CoV-1, MERS, and SARS-CoV-2 coronaviruses, and some avian flu viruses. And some spillover events can result in adaptation to humans as a new stable reservoir. In fact, animals probably transmitted most of the pathogens that are presently exclusive of humans sometime in the past. With prolonged exposure, permanent host-microbe associations may be established, resulting in co-adaptation and even integration of the microbe genome in the human genome, as is the case of retroendogenous viruses. The closer the two species are in genetic terms, the easier it is for microbes to overcome the biological barrier to produce successful spillovers. For this reason, other mammals are the main source of zoonotic agents for humans.
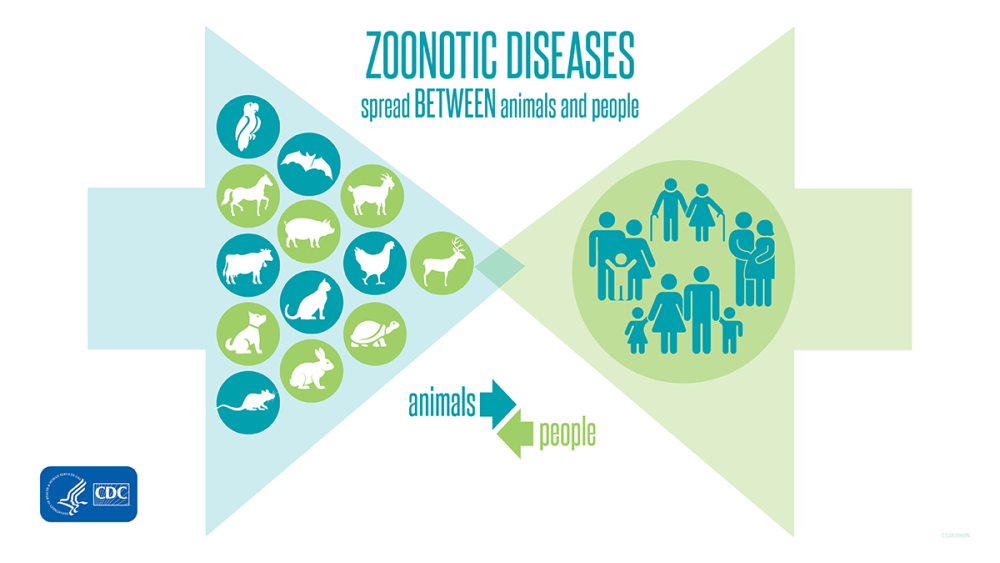
Figure 4. The majority of human diseases and infections originate in animals. Image by CDC (page archived 2021).
Zoonotic spillover has increased in the last 50 years, mainly due to farmlands being influenced by changing environments through removing forests and jungles, changing wildlife habitats, and increasing land use for factories. Animals can even be healthy while carrying disease, making such diseases common both in the United States and around the world.
The Wild Side of Life: Displacement of Host “Cargo”
Zoonosis is an infectious disease caused by a pathogen that has jumped from non-human animals (usually vertebrates) to humans. Animals carry microbes—some “good” and some “bad.” The sylvatic (referring to the occurrence of a subject in or affecting wild animals) cycle, also known as the “wild” transmission cycle, is part of the natural transmission cycle of a pathogen or parasite, yet a fraction of the pathogen’s lifespan is spent cycling between wild animals and vectors. Humans are usually an incidental or dead-end host infected by a vector. The displacement and modification of the virus correlates with how sylvatic diseases become urban diseases. There is “spillover” of the virus, bacteria, or parasite (“host cargo”) from one population to another.
In creation microbiology, microbes (viruses, bacteria, fungi, and parasites) were originally designed in restricted places, but after sin’s curse, they spread to other places and began to cause disease. Sometimes, a mutation in DNA or RNA causes disease. Other times, genetic information gets lost, added, or dislocated. The modification of genetic information and displacement in microbes usually lead to its pathogenicity. Displacement is actually the horizontal transfer of genes, pathogens, and parasites to a different location in nature, often where the sylvatic (or jungle) cycle intersects the urban (domestic) cycle.
Table 2. Definitions (modified CDC 2021)
Zoonosis is an animal infection transmissible to humans.
In epidemiology, a disease vector is any agent which carries and transmits an infectious pathogen into another living organism; agents regarded as vectors are organisms, such as intermediate parasites or microbes.
Sylvatic (jungle) refers to the occurrence of a subject in or affecting wild animals. The sylvatic cycle is the fraction of the pathogen’s life cycle between wild animals and vectors. Humans are usually an incidental or dead-end host, infected by a vector.
In the “domestic” or “urban” cycle, the pathogen cycles between vectors and non-wild, urban, or domestic animals; humans may have differing infection rates from these cycles due to transmission efficiencies and environmental exposure levels.
Displacement: Microbes were originally designed to perform beneficial functions in designated places, but after Adam’s fall, they spread to other places and became problematic. Horizontal or lateral transfer of genes includes pathogenicity islands, transduction, or “spillover.” The viruses, bacteria, fungi, and/or parasites are out of their originally created place. In addition, there is a difference in the immune system among animals and humans.
Spillover infection. Microbes and parasites can occasionally move from one species to another in what is called a spillover event. Such transmission can also be known as a spillover infection or pathogen spillover from a wild host (sylvatic or jungle) to a domestic (or urban) host.
Table 3. Fast Facts About Emerging Diseases
Emerging infectious diseases are those that have recently appeared within a population or geographic range and are considered to rapidly increase. They can be caused by the following:
- Previously unknown infectious agents
- Known agents that have spread to new locations or populations
- Known agents whose role in disease has been unrecognized
- Reemergence of agents whose role in disease had significantly declined in the past but has reappeared (reemerging infectious disease)
- Transmission via proximity between the primary host and the secondary species host—also requires the infectious agent to break through the barriers that would typically prevent spillover events, including circumventing the inherent incompatibility between the agent and its new species and overcoming the new host’s immune response
- After transmission from one host species to another, the successful transmission of a microbe within members of its new secondary host (during this process, the infectious agent attains an increased disease incidence and new hosts are infected at an increasing rate)
Malaria
Malaria is primarily a vector-borne disease in which insects carry it from one host to another. As previously discussed, Plasmodium do not cause disease in some birds but rather are an immune stimulant (Gillen and Sherwin 2013). In humans, however, it causes severe disease. Most human malaria is caused by four “classic” different species of Plasmodium: P. falciparum, P. malariae, P. ovale, and P. vivax. Because vectors are not hosts they belong to a different ecological category from reservoirs; and they experience the presence of a pathogen in different way. Transmission of most malaria parasites from mosquito to human is usually not considered spillover; it is considered vector borne (Quammen 2013). Vectors seek hosts because they need resources (e.g., blood). Reservoirs do not seek spillover. Spillover happens accidentally, and the reservoirs do not gain from it. Therefore, most human malaria (the four traditional species) are not zoonotic because they only infect humans. Most species of monkey malaria infect only monkeys; most species of bird malaria affect only birds. However, the exceptions may be Plasmodium knowlesi, P. cynomolgi, and P. simium. These malaria diseases appear to be zoonotic from monkey to man (fig. 5).
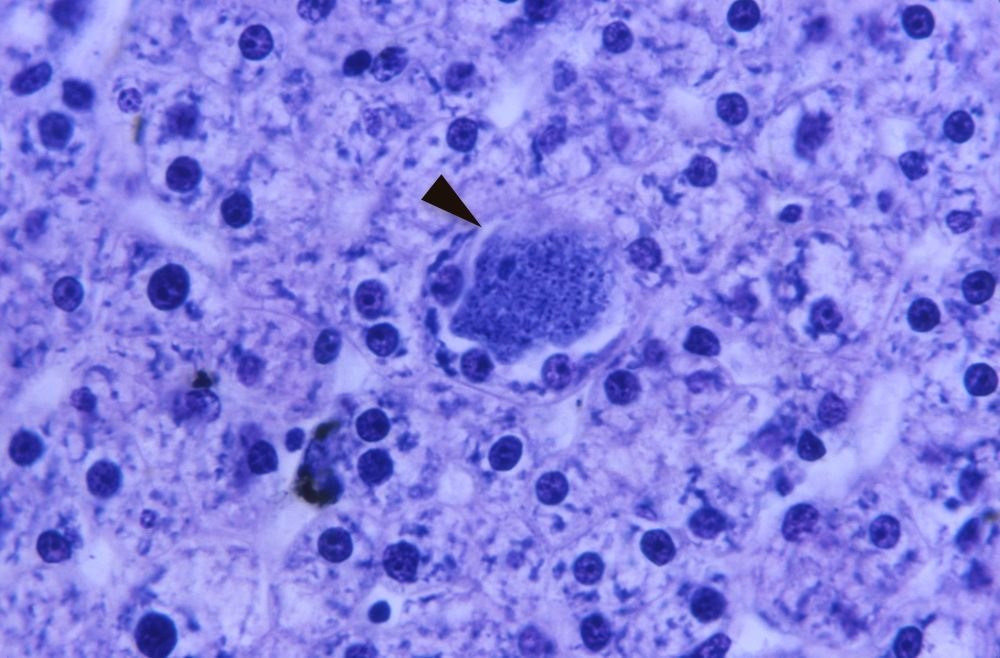
Figure 5. “Monkey” malaria now infects humans. Photomicrograph of rhesus monkey liver tissue infected with P. cynomolgi under 500X magnification. The enlarged hepatocyte (arrow) contains numerous merozoites. Image by CDC/Clinton Smith via CDC.
Monkey-to-Man Malaria Changes
Long-tailed macaques are understood to be the natural host for five Plasmodium species: P. knowlesi, P. fieldi, P. coatneyi, P. cynomolgi, and P. inui. Of these non-human primate species, P. knowlesi and P. cynomolgi have caused an increase in human infections, which were once thought to be extremely rare. One of the earliest outbreaks of P. knowlesi in humans occurred in 2004 in Borneo. Since then, human infections have been reported across nearly all the Southeast Asian countries, making P. knowlesi the fifth Plasmodium species to cause malaria in human hosts (Roberts and Janovy 2013). Recent advances in molecular technology have significantly contributed to the current understanding of Plasmodium distributions as well as the close ancestral relationship between non-human primate parasites with human malaria parasites. Non-human primate parasite spillover into human populations has serious implications, including the potential to challenge eradication efforts. Therefore, an understanding of this transition of certain Plasmodium species from monkeys to humans is vital (fig. 6).
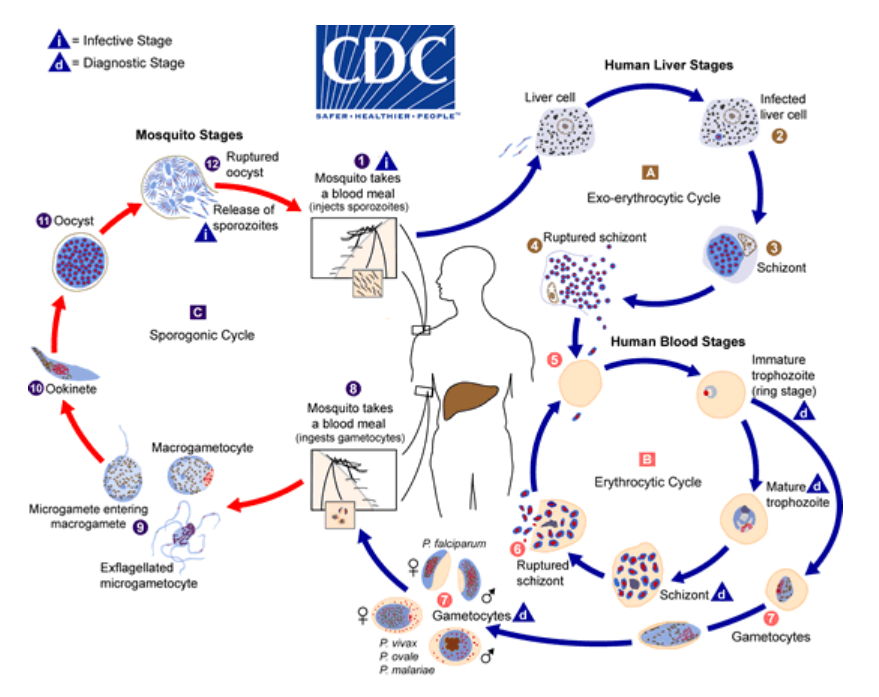
Figure 6. The life cycle of Plasmodium parasites and zoonosis of malaria. The primary host and transmission vectors are the female mosquito. Humans and other vertebrates are secondary hosts. The infected mosquito carries the disease from human to human or vertebrate to vertebrate, while the infected human transmits the parasite to the mosquito. Image by CDC (page archived 2021).
Yoshimasa Maeno et al. (2015) focused on analyzing the species composition of malarial sporozoites infecting the salivary glands of Anopheles dirus. The goal was to determine the Anopheles dirus’ potential role as a bridge vector of Plasmodium from monkeys to humans. Anopheles mosquitoes were collected from a forest fringe area in Vietnam, and the Plasmodium species of the salivary glands were identified using PCR. The six identified species, in order of prevalence, were P. vivax, P. knowlesi, P. inui, P. cynomolgi, P. coatneyi, and P. falciparum. Additionally, 33% of the infected mosquitoes had multiple infections, with most of them being a combination of P. vivax with one or more of the non-human primate species (Maeno 2015). These results suggest that humans in this area of Vietnam are commonly inoculated with both human and non-human primate forms of Plasmodium parasites. This in turn fosters the emergence of novel zoonotic malaria.
Giardiasis
Giardia cause little harm in beavers and some animals; some Giardia mice strains G. muris direct their microbiome (Gillen and Sherwin 2017). Giardia acts as a gut ecosystem engineer, indirectly modulating the availability of resources to other species in the same environment and excreting novel waste products such as lipids or amino acids that can be metabolized by good, enteric bacteria (Gillen and Sherwin 2017). However, when displaced to humans and pets, it can cause disease, as seen in Virginia. One more potential cause to the resurgence of the parasite is the high population of beavers in Virginia (fig. 8). Watersheds, land areas that channel rainfall to creeks, streams, and rivers, are a favorite habitat of beavers. Therefore, the number of watersheds in Virginia would reflect the number of beavers in the state. We have witnessed a booming population of both beavers and Giardia in the James River watershed—the longest and largest one (Gillen 2021). Even as Giardia has become common among beavers, we have not witnessed evidence of diarrhea in the beavers. It is simply part of its normal microbiota.

Figure 7. Contaminated drinking water containing Giardia from beavers can cause Giardiasis in humans. Image by Bob Greenburg/Yellowstone National Park via Wikimedia.
Giardia mainly attaches in the upper GI tract of a wide variety of vertebrates, but it exhibits minimal—if any—disease in some animals. Although Giardia can be pathogenic, some strains colonize the gut with no malady. This parasite is not invasive and affects only the small intestine and may function in non-parasitic, possibly mutualistic ways. Giardia may influence the microbiome, enhancing digestion in certain animals and possibly shifting ratios of bacteria from anaerobic to aerobic (fig. 7). Giardia may play a role in host metabolism and provide nutritional enhancement via its association with enteric bacteria, like E. coli (Gillen and Sherwin 2017).
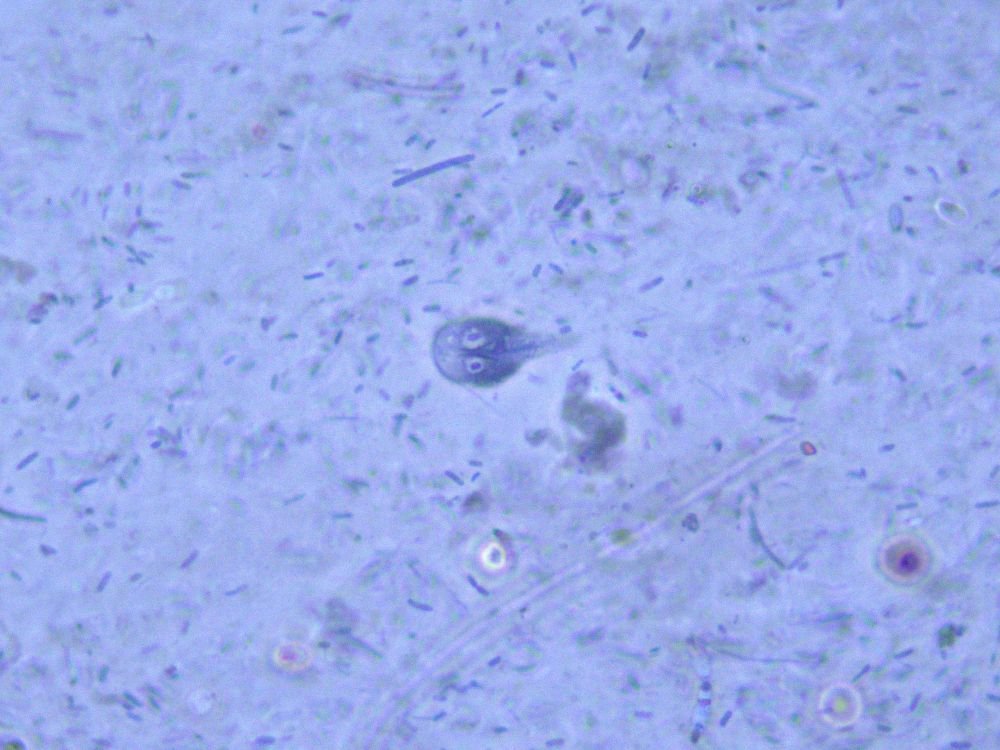
Figure 8. Giardia lamblia under 1000X magnification using a Leica phase contrast microscope. Image by Alan L. Gillen.
Evidence of Displacement
Most E. coli strains are highly adapted to living in humans and only rarely in other animals. E. coli O157:H7, however, lives primarily in cattle, sheep, and other farm animals. E. coli O157:H7 may be designed to make “toxins” to “help” their animal hosts in some way. Sheep infected with E. coli O157:H7 do a better job of withstanding a cancer-causing virus than sheep without the strain, and in cattle, this strain may help cattle obtain benefits such as increased levels of vitamin K. In any case, E. coli O157:H7 does not appear to harm these farm animals (Gillen and Oliver 2010), but in humans, E. coli O157:H7 is deadly.
The two best examples of displacement and mutation that have experimental evidence are E. coli and trypanosomes. Both have variants that are very good in one host and very bad in humans.
Trypanosoma lewisi is a natural hemoflagellate of rats (Rattus species) and other rodents in all areas of the world and is relatively non-pathogenic for human beings; however, there are many reports of Trypanosoma infections in infants in India where rats are common. The human zoonosis has been well documented in trypanosomiasis and is a point of caution considering that animal reservoirs of a variety trypanosomes are around people, especially children. Arthritis (rheumatoid) and anemia have been observed in other animals and people living in developing countries. Dr. David Lincicome of Howard University showed that food intake, vitamin uptake, and body weight changes of rats inoculated with Trypanosoma showed significant increases over the uninoculated controls (Lincicome and Sheppersox 1963). Such research lends support to the creation hypothesis that trypanosomes were mutualistic members of the “normal” microbiota in the pre-fall world. In fact, researchers concluded that the trypanosomes coadapted, bringing more than just tolerance but also a benefit to both trypanosome and the “infected” rodents, much like certain strains of E. coli in mammal GI tracts today.
Lyme Disease
In 1975, it was found that bacteria injected by an infected tick multiply and spread across the skin, then disseminate into body tissues in the bloodstream. The immune reaction produces tissue damage. In the 1970s, this disease became known as Lyme disease.
In Lyme Disease, white-tailed deer are the “normal” definitive host (final host) for the Ixodes (fig. 10) tick (hence the common name “deer” tick). White-footed mice are the typical reservoir hosts, serving as a source of infection for humans or another species. But it is not until the tick attaches and infects humans that the Borrelia bacteria multiply by the billions, causing disease in humans. The displacement of Borrelia from their natural hosts is what leads to major disease issues (figs. 9 and 11).
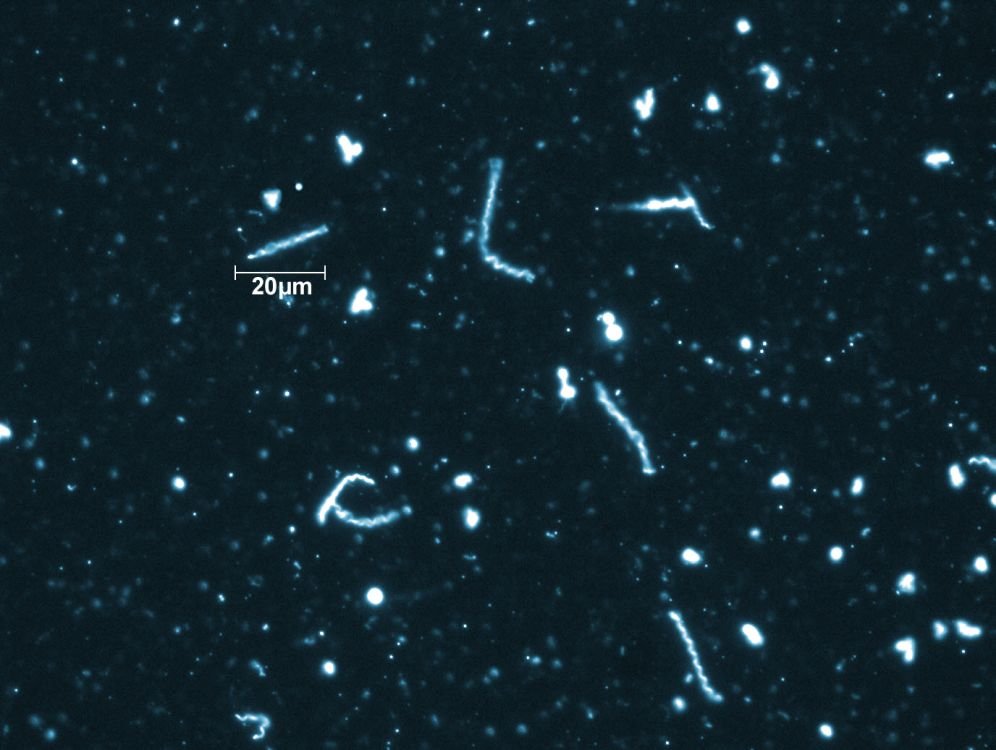
Figure 9. New bacteria variant of Lyme disease. B. mayonii spirochetes under 400X magnification using dark field microscopy technique. B. mayonii was discovered in 2016 and causes diffused rashes rather than the “bull’s eye” rash caused by B. burgdorferi. Image by Adam Replogle via CDC.

Figure 10. The blacklegged tick Ixodes is a vector for B. burgdorferi, which causes Lyme disease. Image by James Gathany via CDC.
Figure 11. Global examples of emerging and reemerging infectious diseases. Image by Anthony Fauci via Wikimedia.
Lyme Disease is the top zoonotic disease in the US, and it appears that both displacement and mutation explain its pathogenicity. Studies have found that there are nonpathogenic Borrelia found in ticks and that it is a part of the normal microbiome benefitting the tick (Anderson, Barthold, and Magnarelli 1990).
Mutation of the Borrelia bacteria have added to complexity of Lyme disease. For a long time, it was believed that one main species caused Lyme disease until B. mayonii was discovered in 2016. Through the research that has been done by the Mayo Clinic in areas like Minnesota, Wisconsin, and North Dakota, this new species, B. mayonii, was found in only 6 of the 9,000 samples taken from the residents (CDC 2019). This showed that it was genetically distinct from B. burgdorferi. B. mayonii appears to be limited to the midwestern United States, as roughly 25,000 samples taken from the Northeast and Mid-Atlantic region where the most common form of Lyme disease is found did not contain B. mayonii (CDC 2016). Like other microbes undergoing mutation and variation, we also see mutation and variation in Lyme disease today (CDC 2016). More details about the origins of Lyme disease and ticks can be found in an upcoming paper.
Summary and Conclusions
Displacement and mutation explain the origin of most infectious diseases in our modern world since the curse described in Genesis. Contagion has naturally followed. Plagues, pestilences, and epidemics have been with us since ancient times, at least since the time of Job and early Israel (plagues of Egypt). Many of these have an animal connection (fig.11). New and re-emerging diseases frequently occur because of human contact with animals. A pandemic is an outbreak of a disease that occurs over a wide geographic area and affects a high proportion of the population. Pandemics like the Spanish flu (1918–1920 influenza) and bubonic plague described in The Genesis of Germs (Gillen 2020) are uncommon. The last cases of Spanish flu occurred in December 1919 and a few into January 1920. A century later, COVID-19 became the next global concern. There were early warnings by Chinese physicians in November 2019 that they were observing a SARS-like disease in patients. Their warnings were ignored by their government, and by late December 2019 an epidemic of an acute respiratory-tract illness was widespread going into the new year. Perhaps ironically, 100 years after the Spanish flu outbreak, the world was shaken again.
Plagues, pestilences, and epidemics have been with us since ancient times, at least since the time of Job and early Israel (plagues of Egypt).
When terrible diseases come our way, believers in Jesus Christ can take refuge in the shadow of the Almighty. Psalm 91 says, “Surely He shall deliver thee from the noisome (deadly) pestilence.” Although Christians are not exempt from terrifying diseases, it is true that they can and will find refuge in their Creator. Christians are not called to fear these diseases but to study and apply known biblical principles (such as quarantine, hygiene, and giving or obtaining medical help as needed) in times of crisis.
There will always be new variants due to mutation, reassortment, modification (fig. 12) until Christ returns. Deadly plagues are not new to man. We read of deadly pestilence and infectious disease outbreaks throughout the Bible. In the Old Testament, we read about laws that Moses gave principles for controlling disease, such as quarantines and cleaning surfaces with hyssop soaps. In Exodus 15:26, Jehovah Rapha states that He would put none of these diseases upon those who obeyed him. It is interesting that when God’s people have followed these health principles, fatalities have been minimized. For example, in 1665, bubonic plague was negligible among Jews. The bible speaks of plagues (Exodus) and pestilences from times of old to the end (Revelation). The genesis of germs will stop, and eventually there will be an exodus of germs (Revelation 22).
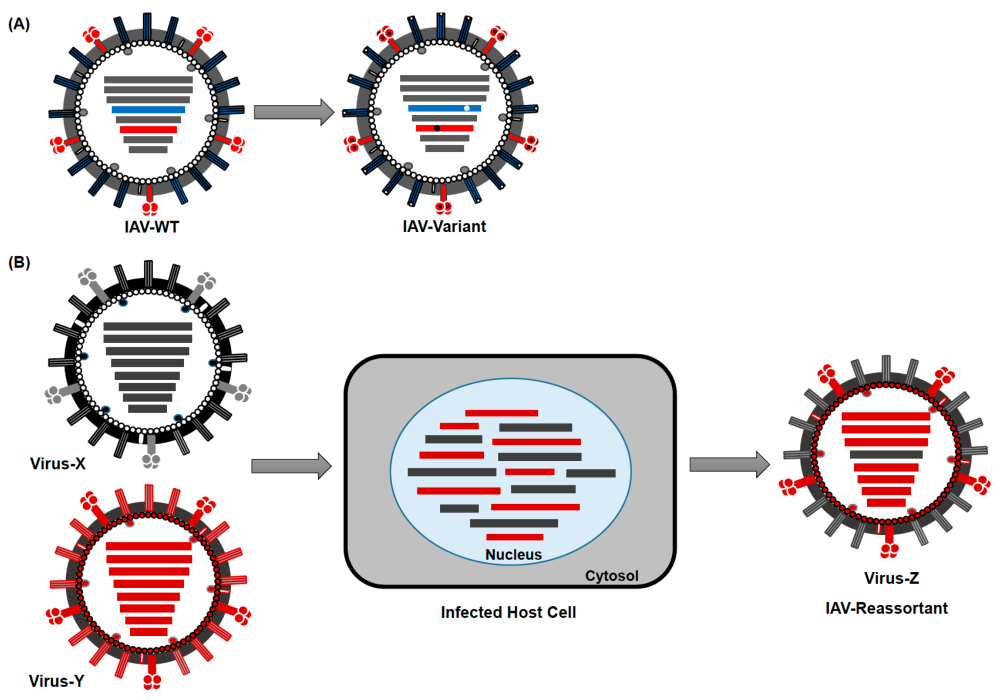
Figure 12. Viral diseases change by mutation, reassortment and modification. These changes occur through mechanisms such as: (A) Antigenic Drift and (B) Antigenic Shift. Image by Ahmed Mostafa, Elsayed M. Abdelwhab, Thomas C. Mettenleiter, and Stephan Pleschka via Wikimedia.
References
Anderson, J. F., S. W. Barthold, and L. A. Magnarelli. 1990. “Infectious but Nonpathogenic Isolate of Borrelia burgdorferi.” Journal of Clinical Microbiology, 28, no. 12 (February 2, 2021): 2693–2699. https://doi.org/10.1128/jcm.28.12.2693-2699.1990.
Centers for Disease Control and Prevention. 2019. “What You Need to Know About Borrelia mayonii.” Last reviewed September 12, 2019. Archived March 20, 2021, at https://web.archive.org/web/20210320012530/https://www.cdc.gov/lyme/mayonii/index.html.
Centers for Disease Control and Prevention. 2016. “New Lyme-disease-causing bacteria species discovered.” Last reviewed February 8, 2016. Archived March 14, 2021, at https://web.archive.org/web/20210314164600/https://www.cdc.gov/media/releases/2016/p0208-lyme-disease.html.
Centers for Disease Control and Prevention. 2021. Definitions.
Chess, B. and K. P. Talaro. 2021. Talaro’s Foundations in Microbiology: Basic Principles, 11th Ed. Boston, Massachusetts: WCB McGraw-Hill.
Cowan, M. K. and H. Smith. 2021. Microbiology: A Systems Approach, 6th Edition Boston, Massachusetts: WCB McGraw-Hill.
Gillen, A. L. 2020. The Genesis of Germs: Disease and the Coming Plagues in a Fallen World, Revised Edition. Green Forest, Arkansas: Master Books.
Gillen, A. L. 2021. “Liberty University Researchers Study the Effects of Rainfall on Giardia and E. coli in the James River Watershed.” Virginia Scientists XXVII, no. 1 (Spring): 6. https://vacadsci.org/wp-content/uploads/2021/03/VA-Scientists-2021-Spring-issue.pdf.
Gillen, A. L. and J. Conrad. 2020. “The Genesis of SARS-CoV-2 virus and the Origin of the COVID- 19 Pandemic.” Answers In-Depth (June 17). https://answersingenesis.org/coronavirus/genesis-sars-cov-2-virus-and-origin-covid-19-pandemic/.
Gillen, A. L. and J. D. Oliver. 2010. “The Genesis of Pathogenic E. coli.” Answers In-Depth (October 6). https://answersingenesis.org/biology/microbiology/the-genesis-of-pathogenic-e-coli/.
Gillen, A. L. and F. Sherwin. 2013. ”The Genesis of Malaria. The Origin of Mosquitoes and their Protistan Cargo, Plasmodium falciparum.” Answers in Genesis (June 19). https://answersingenesis.org/biology/disease/the-genesis-of-malaria/.
Gillen, A. L. and F. Sherwin. 2017. “The Design of Giardia and the Genesis of Giardiasis.” Answers In-Depth (July 19). https://answersingenesis.org/biology/microbiology/design-giardia-and-genesis-giardiasis/.
Johns Hopkins Coronavirus Resource Center. (n.d.). “COVID-19 United States Cases by County.” https://coronavirus.jhu.edu/us-map.
Lincicome, D. R. 1971. “The Goodness of Parasitism: a New Hypothesis.” In Some Aspects of the Biology of Symbiosis by T. C. Cheng, 139–227. Baltimore: University Park Press.
Lincicome, D. R. and J. R. Sheppersox. 1963. “Increased Rate of Growth of Mice Infected with Trypanosoma duttoni.” Journal of Parasitology 49 (February): 31–34.
Maeno, Y. et al. 2015. “Humans Frequently Exposed to a Range of Non-Human Primate Malaria Parasite Species Through the Bites of Anopheles virus Mosquitoes in South-Central Vietnam.” Parasites & Vectors 8 (July 16): 376. https://doi.org/10.1186/s13071-015-0995-y.
Quammen, D. 2013. Spillover: Animal Infections and the Next Human Pandemic. W. W. Norton and Company. New York.
Roberts, L. S., J. Janovy Jr., and S. Nadler. 2013. Schmidt and Roberts Foundations of Parasitology, 9th ed. Boston, Massachusetts: WCB McGraw-Hill.
Answers in Depth
2021 Volume 16
Answers in Depth explores the biblical worldview in addressing modern scientific research, history, current events, popular media, theology, and much more.
Browse Volume
Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis

