The Genesis of Malaria
The Origin of Mosquitoes and Their Protistan Cargo, Plasmodium falciparum
Abstract
Malaria is caused by the parasite belonging to the genus Plasmodium; however, creation biologists maintain this organism was not always parasitic. Plasmodium is probably a degenerate form of algae. Mosquitoes, the vector of Plasmodium, were probably designed to be pollinators, not parasite vectors. In this article, we present both the evolutionary and creation explanation for the origin of malaria with a mention to its vector, the mosquito.
The purpose of this article is to provide a reasonable explanation for the genesis of malaria. Microbiology and parasitology research based on the creation paradigm appears to provide some answers to these puzzling questions regarding the Plasmodium “kind” (Family Haemosporidae). Although we cannot be dogmatic (beyond the biblical text) regarding details of Plasmodium’s origin during Creation Week, we believe that a reasonable extrapolation from Scripture and biological data can be made about the nature of protozoans in a fully mature creation.
Keywords: malaria, mosquitoes, Plasmodium falciparum, parasitism, genesis of germs, apicoplast, ancient algae, symbiosis, biomatrix, organosubstrate, microbiology, parasitology
Malaria is one of the oldest diseases that have continuously plagued mankind. We may have ancient records of malaria-like illness from 5000 years ago in China, 3000 years ago in Egypt and the Middle East (Shah 2011; Perry 2011).1 There are even mentions in Scripture of such an illness (see below). Regardless, malaria is not just a scourge of the ancient world, but it is serious parasitic disease today. According to the World Health Organization 2012 report, there were 219 million cases of malaria that caused about 660,000 deaths worldwide, mostly children. Currently 600 million people in 109 countries are at a high risk of contracting malaria.
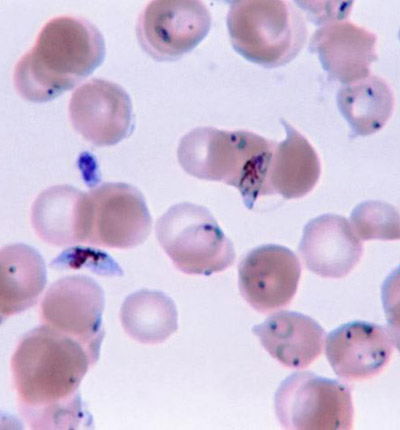
Fig. 1. Plasmodium falciparum photo, Wikimedia Commons
Yet, we read in Genesis 1:31 that God made everything “very good.” If everything that God made was good, where did malaria come from? What is the origin of this frightening parasite, Plasmodium falciparum (Fig. 1)? Where does malaria fit in to the creation account? Was the malaria parasite created along with the rest of life in the first week of Creation, or was it created later, after the Fall? Is malaria a result of the Curse? These and other questions have been asked by creation biologists (Francis 2003; Gillen 2007) and their answers may surprise you. Diligent research by past creation biologists such as Leeuwenhoek, Pasteur, and Ross were blessed by God as He revealed (Psalm 139:17a) to them critical insight into His creation (Gillen and Sherwin 2008).
Pre-Fall World and Characteristics of Parasites
Before answering these questions, some background is needed regarding the Pre-Fall world, normal microbiota (i.e. bacteria, fungi, and protozoans), and mosquitoes in particular. Where do the malaria parasites fit into God’s “very good” creation? Most creation biologists believe that God created (Genesis 1 and 2) all microbes and protists as very good forms of life (Francis 2003; Gillen 2008). Even today, a vast majority of bacteria, fungi, and protozoans are beneficial to man and nature. Many larger life forms live in a beneficial relationship with smaller life forms that biologists call symbiosis (Greek sym; together, bios, life). In general, symbiosis in nature involves a larger host and a smaller symbiont. There are three types of symbiosis in nature. Mutualism is a type of symbiosis where both host and symbiont have a beneficial relationship. Commensalism is a type of symbiosis where one life form is benefited and the other is unharmed. In these two symbiotic relationships, two different organisms live together in harmony. A common pattern observed is one in which a larger organism is interwoven2 with one or more smaller life forms (i.e. microbiome).
Creation biologists would suggest that parasitism is a secondary state in nature. After the Fall, something “bad” happened: some organisms degenerated into parasites. The key to understanding most parasites is that they have a “broken” relationship with their host. In the beginning, most large creatures were designed to live with smaller ones. In contrast, most evolutionary biologists believe that small and large creatures came to associate with each other by chance and some smaller ones progressively emerged to exploit their host. According to Darwinists, animal complexity evolved through time and chance where some smaller animals emerged as parasites to larger animals through natural selection (i.e. upward, onward evolution).
Parasitism is a type of symbiosis where one organism (the parasite) benefits at the expense of the host. Parasites are usually smaller than their host, exhibit a high degree of specialization, and reproduce quickly and in vast numbers in or on their hosts. Classic examples of human parasitism include interactions between man and diverse protozoans (i.e. Giardia and Plasmodium), worms (i.e. tapeworms and flukes), and ectoparasites (i.e. fleas, lice, ticks, and mosquitoes).
The Origin of Plasmodium
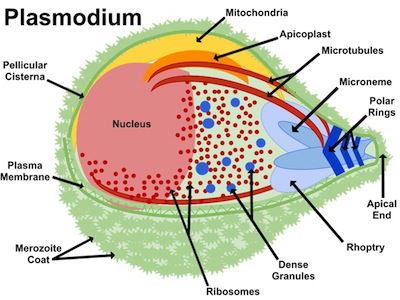
Fig 2. Apicoplast. Plasmodium anatomy figure, Wikimedia Commons
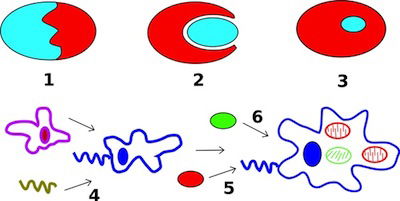
Fig. 3. Secondary endosymbiosis in Wikimedia Commons
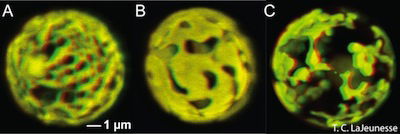
Fig. 4. Algae and Chloroplast. Symbiodinium is a dinoflagellate algae. Its chloroplast is shown in this Confocal microscope photograph. Wikimedia Commons. The reticulated chloroplast structures of (A) Symbiodinium goreaui (type C1), (B) S. fitti (type A3), and (C) S. californium (type E1) imaged in 3-D using confocal microscopy of chlorophyll autofluorescence (LaJeunesse et al. 2010a).
Plasmodium is included with the intracellular parasites in the phylum Apicomplexa. One of the unique features of these parasites is the apicoplast (Fig. 2), a four-membrane-bounded plastid. These apicoplasts3 are similar to the chloroplasts4 found in plants and algae, which may indicate that Plasmodium descended from algae. Its apicoplasts may be the remnants of chloroplasts: organelles designed for photosynthesis. According to Darwinian biologists, Plasmodium originally gained its apicoplasts through endosymbiosis, the process of a cell engulfing another cell and maintaining it internally for its benefit. In fact, the latest evolutionary explanation is that it was a series of endosymbioses, or serial endosymbiosis (Fig. 3).
Evolutionists maintain the Plasmodium gained chloroplasts (Fig. 4) it used for photosynthesis from red algae. Indeed, ten percent of the proteins present in Plasmodium are involved in photosynthetic mechanisms. Although the photosynthetic ability has been lost, the apicoplasts in Plasmodium still have vital functions that resemble biosynthetic pathways in plants. Sometime after the origin of “modern” protists, Plasmodium lost its ability to manufacture its own food and became parasitic to survive. Evolutionists maintain there was a connection regarding algae growing alongside of mosquito eggs and larvae (Shah 2011). Darwinists maintain that the ancestors of malaria living alongside the larvae gradually adapted to living inside the gut of the mosquito and evolved into today’s Plasmodium. After millions of years of evolution, the 250 species of Plasmodium known today emerged (Perry 2011).
The Genesis of Malaria: An Ancient Disease
Alex Perry in his 2011 book, Lifeblood, calls malaria “Original Sickness.” It may be debated if it was the first sickness; but it certainly is one of the earliest of the deadly parasitic diseases. Perry describes malaria as a global scourge, a “driver” of human history, and factor of human adaptation to tropical areas. In China, malaria may have existed almost 5000 years ago. Nei Ching, the 4700-year-old canon of Chinese medicine, mentions reoccurring fevers that enlarged the spleen. Plasmodium falciparum has been found in 40% of Egyptian mummies (dated to 3200 BC) at the Turin Museum in Italy. Malaria may also have killed pharaohs in 1324 BC, stopped Alexander the Great in 326 BC, and influenced thousands of other military campaigns in ancient times (Shah 2011; Perry 2011). These dates may vary slightly from the biblical time frame given by conservative scholars; however, the observation of malaria a few thousands of years ago (not millions) are reasonable and demonstrate that malaria is a very ancient disease. Malaria has no doubt played a role in the lives Jews and early Christians. Although it is not named specifically in the Scriptures, it is possible that two passages in the Bible are referring to malaria. These include: Deuteronomy 28:22 (fever); Matthew 8:14–16 (also parallel in Mark 1:30–31 and Luke 4:37–38) and the fever in Peter’s mother in-law. According to medical doctor and apologist, A. Rendle Short (1955), the most likely Bible passages pertaining to malaria are those telling of the case of fever in Peter’s mother in-law.
The Genesis of Malaria Germs
Given the great destruction of malaria over the centuries, it is valid to ask of its origin. Interestingly, recent electron microscope analysis of Plasmodium has shown that its “apicomplex” organelle (the apicoplast) is actually a degenerate chloroplast. This means very probably that the malaria parasite (Plasmodium) used to be an alga, a free-living, plant-like aquatic protozoan (Fig. 4). It was neither in the blood of man nor mosquito. Apparently, it was created as an autotroph—to make its own food; but sometime after the Fall, it lost its ability to photosynthesize and became parasitic. Now, it causes global misery!
Ancient Algae
Plasmodium’s ancestors were probably extinct protist-like algae with unique traits. It was probably similar to Chromera velia, an endosymbiotic algae of reef corals, Acropora. Some biologists consider Chromera to be a “mutualistic” apicomplexan (an organism with an apicoplast, or of the phylum Apicomplexa, or a protozoan organism having some sort of capability to invade host cells). Evolutionists call Chromera the bridge from algae to apicomplexan. Creation biologists would say Chromera still performs its “pre-Fall” function (photosynthesis) as an apicomplexan. The other apicomplexans, like Plasmodium and its predecessors, lost information, i.e. the ability to photosynthesize. Then, the chloroplast degenerated into apicoplasts.
There appears to be no known specific algae species that fits the description as the specific ancestor to Plasmodium. However, there are several algae kinds that have similarity to that of the malaria parasite. In sexual reproduction, Plasmodium is similar to the common unicellular green algae, Chlamydomonas. They both undergo syngamy, the joining of two haploid gametes to form a diploid zygote. Specific receptors are found embedded in the sex cell membrane and help the male gamete to attach to its partner tightly. These membrane attachments contain species-limited proteins. In its asexual reproduction, Plasmodium is similar to dinoflagellates. They use their hosts to aid in their survival and reproduction as well. Plasmodium is also similar to Crithidia. Both are endosymbionts of Anopheles mosquitoes.
Plasmodium Degeneration and Displacement
Plasmodium is better seen as a product of degeneration, displacement, and devolution; not upward, onward evolution and endosymbiosis, as portrayed by evolutionists. After the Fall of man, these algal-like organisms began to degenerate and became displaced. Like all true parasites, Plasmodium had to rely on a host for shelter and nutrient acquisition. Due to the complexity of the Plasmodium lifecycle and its easy adaption to Anopheles mosquitoes, we hypothesize that, likely as an algae symbiont, it originally had one life stage in the gut of mosquitoes and another outside the mosquito. The algae photosynthetic product may have supplied protein or material needed for females to make eggs.
Plasmodium and The Origin of the Apicoplast
Plasmodium’s organelle, the apicoplast, has the essential role of providing the parasite with chemical precursors for reproduction in its erythrocytic life stage. An evolutionary explanation for the origin of the apicoplast is one of consecutive serial endosymbioses. Photosynthetic eukaryotic alga was the first product of this symbiotic relationship. Another symbiotic relationship was formed when a heterotrophic eukaryote engulfed a relative of photosynthetic eukaryotic alga. Darwinists maintain that a plastid (called a chloroplast in plants) formed as a result of this relationship through endosymbiosis. The plastid grew into its role over time to preserve functions and genes to maintain the host-organelle relationship. The plastid degenerated and was rearranged so that it could no longer photosynthesize, becoming the apicoplast found in Plasmodium today. The rearrangement must have occurred long ago in the evolutionary stage so that it could be where it is today. Some evolutionary scientists suggest the apicoplast may have been the result of a tertiary endosymbiosis.
Creation biologists think that the chloroplast (originally designed by God) degenerated and was rearranged to form the apicoplast (at some point after the Fall). However, it is very unlikely that any endosymbiotic event took place; this would require increasing complexity. The chloroplast’s function changed from photosynthesis to production of chemicals needed to invade a red blood cell. The chloroplast’s photosynthetic product originally may have provided the critical protein needed for normal egg development in mosquitoes. Algae (which contain chloroplasts) have always been seen as producing primarily carbohydrates, but it has recently been shown that some algae produce protein. In fact, there is a company that now sells an “ultra”-premium protein powder from red algae as an alternative to soy and whey protein powder.
The Design of Mosquitoes

Fig. 5. Anopheles gambiae mosquito: CDC photo, Anopheles 11998. This photograph depicts two Anopheles gambiae mosquitoes as the female at the top of the image, was in the process of egg-laying atop a sheet of egg paper. The male is at the bottom of the image. A. gambiae is the principal vector of malaria in Africa.
Of all the insects that God created, it is the mosquito (Fig. 5) that has produced the most death and misery in human history. If God made everything good, then where do they fit in? Male mosquitoes are harmless, designed with mouthparts that can only feed on plant juices and nectar. Only females need the protein found in a blood meal to produce eggs. Most female mosquitoes have a large proboscis and stylets for piercing skin. Females feed mostly at night on small mammals, primates, and humans. They find their hosts by exhaled carbon dioxide located by the mosquito’s sensitive antennae. By probing the skin they locate a capillary and begin to feed. The need for blood is a reproductive (gamete formation) issue, more so than a “survival” issue.
Mosquitoes as Pollinators
In today’s world, some tend to think of mosquitoes as God’s “mistake” or a vicious outcome of the Curse. After all, they are not only the deadly vectors of malaria, but also West Nile encephalitis, yellow fever, dengue fever, elephantiasis (lymphatic filariasis) and many more viral (and some parasitic) diseases. However, creationists maintain they did not carry these diseases before the Fall. Not surprisingly, there is much evidence of intelligent design in their anatomy, physiology and purpose in the natural world. Like bees, they were probably designed as pollinators. Mosquitoes pollinate goldenrod, grasses, and different types of the large group of orchids even today. After the Curse, it appears the nutritional needs of mosquitoes changed.
In his 8–9 day life cycle, the male mosquito must find a mate. During this time he takes in nectar through his designed proboscis. His wings beat silently and his antennae have been specially equipped with special sensory cells equipped with hairs. The female’s wings are designed to beat with an intensity that can move these hairs up to 10 inches away. This stimulates the mating process and associated physiological changes. The female carefully stores the male’s sperm in a special sac in her body to later fertilize eggs.
Each blood meal ensures the female mosquito will have a healthy batch of eggs. She embarks on her hunt for a blood meal using her ultra-sensitive antennae. Once she locates her victim, she uses her infamous proboscis (designed with microscopic ridges to reduce pain) to inject her numbing and anticoagulant juices. Within this structure are also two tiny spears or stylets, and a “saw” for cutting through skin. A syringe connected to a pump in her head allows her to draw up to three to four times her body weight in blood (seen in her semi-transparent abdomen). Previously, the originally good design may have enabled mosquitoes to penetrate into thick plant tissues or even beneath soil to reach the roots of plants.
Insight from Other Protistan “Cargo”
Creation biologists maintain the Protistan “cargo” of mosquitoes did not originally need blood to survive and reproduce and that plant extracts supplied the nutritional needs. For example Haemosporidia (like Plasmodium) and trypanosomes may have utilized a protein similar to leghemoglobin5 (a plant hemoprotein). In today’s world, mosquitoes cannot reach the leghemoglobin found in the roots of soy plants. Perhaps a similar protein once existed in an extinct plant, or mosquitoes once had a way of accessing this protein found in soybeans.
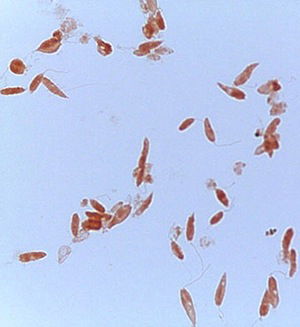
Fig. 6. Crithidia, Malachite Green and Congo Red stain. Note the Crithidia’s organelles. Alan L. Gillen and Zachary Rhodenizer image
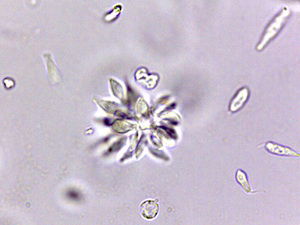
Fig. 7. Crithidia in rosette formation. Rosette formation is due to rapid binary fission in circular and rose-like appearance. Plasmodium is noted for its rosette formation and it has clinical implications – it clogs blood vessels and causes severe bleeding. Alan L. Gillen image
Crithidia fasciculata is a trypanosome (parasitic protozoan, Figs. 6 and 7) of the phylum Euglenozoa and has a single host, the mosquito Anopheles (first described in 1902). Since its initial discovery, C. fasciculata has been found to infect many species of the nearly 450 species of Anopheles. Over the past 100 years Crithidia fasciculata has been used to investigate protozoan metabolism. Early studies showed that Crithidia could be grown in peptone water with blood added. Since the 1960s papers were published describing cultural conditions and organic requirements of this parasite. Although Crithidia fasciculata has been used for metabolic studies, the research was hampered by inconsistent growth using traditional media of the 20th century. We therefore undertook a systematic search for the chemical and physical conditions that would permit rapid and abundant growth of the organism (Fig. 7).
At Liberty University, Gillen (2013) describes modified chemically-defined media for Crithidia fasciculata which allows for consistent analysis of its nutritional and metabolic patterns. Using these improved conditions we have examined growth factor requirements of Crithidia fasciculata. Data was collected on Crithidia’s growth factors using a spectrophotometer. The conditions for luxuriant axenic (free of contamination) cultivation of this trypanosome using chemically-defined media were determined in a parasitology class. Crithidia is a hardy organism and survives in a variety of media. It grows and reproduces best in a blood-enriched medium or in trypticase soy broth (TSB) with added folic acid and vitamin B12. TSB + folic acid + B12 (plant-based material) is a reasonable substitute for blood. Reproduction as shown by numerous rosette formations indicates Crithidia can reproduce well in plant-extract environment if the right amino acids and vitamins are supplied.
In laboratory experiments, Crithidia fasciculata will survive longer on a blood agar broth than any Crithidia media (including the vitamin-enriched TSB + B12 + folic acid). Mosquitoes and their “cargo” (whether Crithidia fasciculata or Plasmodium) can survive and reproduce on either blood or plant-extracts. But it appears blood can provide an extended sustainability. Mosquitoes would possibly require more plant extracts for their nutritional requirement. Compared to nectar and plant extracts, blood is a rich, scarlet soup of proteins. Mosquitoes feeding on blood receive a rich source of protein and nutrients for egg production. However, there is a cost to the mosquito. Blood is heavy and makes the slow mosquito vulnerable to predation. Once mosquitoes obtain blood, they are also more vulnerable to be “swatted” by a human or animal (as opposed to plant) host, making this slower mobility even more of a risk.
Blood agar added to TSB provided a media for rich growth but plant-based trypticase soy broth with vitamins (folic acid and B12) provide similar high growth and reproductive yields. Perhaps Plasmodium and Anopheles with a mammalian intermediate host of today had its protein demands uniquely fulfilled by nutritious plant-based products in the past.
Mosquito Microbiome
One practical consideration in studying mosquito “cargo” is altering its microbiota to inhibit pathogenicity. Perhaps in the mosquito’s originally designed microbiome, bacteria blocked pathogens and parasites from becoming established in their hosts. Recent research on Wolbachia, a genus of bacteria that “colonize” insects (including mosquitoes) and nematodes, indicates it can alter the pathogenic properties of these invertebrates by ecologically displacing them. These intracellular bacteria generally form symbiotic relationships with their hosts. When Wolbachia colonize and “infect” mosquitoes, such as Aedes aegypti, the transmission of dengue fever is halted. This research has been done for about a decade in an attempt to control Flaviviridae (yellow and dengue fever) infections. In recent studies (Enserink 2013), scientists have now been able to infect Anopheles stephensi with Wolbachia and alter the transmission of malaria. Anopheles stephensi is a key malaria vector in South Asia and the Middle East. Perhaps in the original design of mosquitoes, bacteria such as Wolbachia were part of its normal microbiome, thus inhibiting the effect of Plasmodium (malaria) or viral infections.
The key seems to be ecological residency. People normally do not get fungal skin infections because normal bacterial biota are already there taking up that ecological niche and preventing fungi from getting a foothold. The sciences of microbiology and parasitology use biological controls and apply ecological principles. For example, biologists pit microbes against one another when they add a benign resident like Wolbachia to a mosquito vector. Once Wolbachia moves in and establishes residence (i.e. take up that ecological niche), then parasites like Plasmodium cannot occupy this space, and they are controlled. Biologists are currently utilizing nonpathogenic bacteria to counteract and control virulent microbial pathogens and parasites. Results indicate they can now quell a variety of infectious disease. According to a survey Wolbachia species are found in 65% of all insect species, including 28% of mosquito species. In many cases, hosts are protected from pathogens and parasites, including nematodes and viruses, as well as those that cause malaria. Wolbachia may well boost the insect immune system, priming it to attack virulent microbes and parasites (Weiman 2013). In a pre-Fallen world, Wolbachia and Plasmodium may have co-inhabited mosquito guts and each controlled the overgrowth of the other. We do not know the original, complex ecological web of mosquito gut microbiota, but this line of research may provide clues to the original design. The “balance” (or imbalance) of numbers in the normal microbiome may be a key to the puzzle of malaria’s origin.
The Origin of a Complex Life Cycle
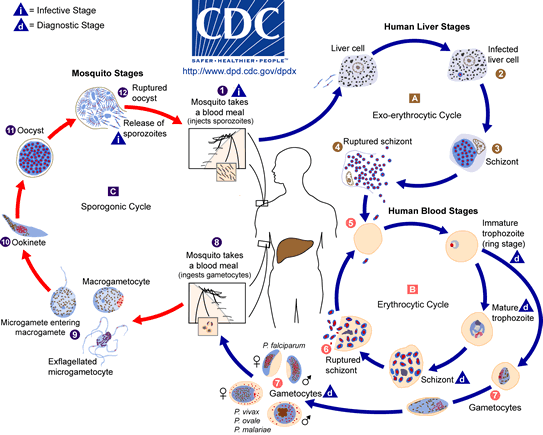
Plasmodium life cycle; CDC
All species of malaria pass through the same complex life cycle involving transmission between intermediate and definitive hosts (Fig. 8). This intricate life cycle makes it difficult to accept that random evolution could produce such step-by-step complexity. Evolutionary biologists must imagine a series of consecutive, beneficial mutations and changes to develop this complex life cycle spanning many hundreds of millions of years. This hypothesis is suggested because Plasmodium must transform itself into more than seven different stages that vary in physiology and morphology. The physicochemical conditions demand such variation. Plasmodium must quickly adapt to the mosquito’s and vertebrate’s physiology rapidly in order to survive and reproduce. A simple version is described in the text below.
There are two distinct phases: in the mosquito (the definitive host), where Plasmodium undergoes sexual reproduction; and in the human body (the intermediate host), where Plasmodium undergoes asexual reproduction. A diagram summarizing the life cycle is given in Figure 8. The life cycles of the malarial parasites have three important stages: the sporozoite, the merozoite, and the gametocyte. Each is a period or stage in malaria. The mosquito sucks human blood and acquires gametocytes that will later produce male and female gametes. (The mosquito is biting a person infected with malaria to acquire this parasite stage.) The male gamete (sperm) fertilizes the female gamete (egg) to produce a fertilized egg (the sexual cycle). This motile, elongate zygote—called the ookinete—penetrates the stomach lining of the mosquito, becomes an oocyst, and undergoes sporogony (cell division occurs and asexual sporozoites form). Tiny sporozoites develop in the oocyst, which ruptures, and the sporozoites migrate to the mosquito’s salivary glands. When an Anopheles carrying the infective stage of Plasmodium (sporozoite) bites a human, it injects these into the blood stream.
The blood carries the sporozoites to the liver, undergoing schizogony in liver cells and producing thousands of progeny called merozoites. Merozoites enter the bloodstream and infect red blood cells. The young trophozoite looks like a ring in which the nucleus and cytoplasm are visible. This is called a ring stage. The ring stage enlarges and divides repeatedly, and the red blood cells eventually rupture and release more merozoites. Upon release of the merozoites, their waste products, which cause fever and chills, are also released. Most of the merozoites infect new red blood cells and perpetuate the cycle of asexual reproduction. However, some develop into male and female sexual forms (gametocytes), which infects the mosquito as described above.
Possible Role in the Vertebrate
In regard to the creation model, it is possible the life cycle of Plasmodium—from the beginning—had a non-parasitic cycle in some vertebrates. Even today, most Haemosporidia cause little harm to their host in the wild (Roberts, Janovy, and Nadler 2013, p.143). Some species of Plasmodium causing avian malaria may even stimulate the immune system in juveniles and cause a more vigorous response in adults. This suggests Plasmodium was designed as an immune modulator in the vertebrate host. In a pre-Fall world devoid of disease, the immune system could have regulated the normal human microbiome and been involved in the recycling of old cells. But, after the Fall, its displacement (perhaps by zoonosis6) caused the disease condition we call malaria. This immune modulation ability would explain Plasmodium’s remarkable capacity to change its physiology quickly from a mosquito (an invertebrate host) to a vertebrate. Such a sophisticated life cycle outside the mosquito could not evolve through random mutations. It is likely that asexual stages were already a part of some vertebrates at creation. For example, several stages such as the merozoites show an amazing adaption and tolerance to the host defenses and are immunoprivileged (the immune system does not attack it).
One possible non-evolutionary explanation regarding Plasmodium may have been to stimulate or “fine-tune” the immune system in birds (again, this still would have been necessary in a pre-Fall world). For example, Plasmodium reticulum and P. circumflexum in some birds show neither pathogenic nor invasive characteristics. Juvenile birds exposed to Haemosporidia show a robust immune response to pathogenic threats such as virulent microbes later in life. Infected individuals showed elevated leukocyte (i.e. eosinophil) counts, but few symptoms of avian malaria. The elevated eosinophil count means that Plasmodium is active in stimulating the immune system, but not causing any harm. The stimulation in these birds may prevent them from getting a more serious disease later in life (Roberts, Janovy, and Nadler 2013).
One avian Plasmodium (e.g. P. gallinacean) does not harm ducks but causes avian malaria in other birds. Plasmodium gallinacean devastates chickens. In ducks and canaries, there appears to be a tissue-blood barrier to Plasmodium. Plasmodium affects the reticuloendothelial system (RES)7 but does not actually invade the blood cells. Plasmodium gallinacean are abundant in their cryptozoites and metacryptozoites life stages in ducks, however, there is no paratesemia—no blood invasion—no disease (Smyth, 1976). It may be that in the beginning, avian Plasmodium were designed to stimulate the immune system without harming it and to help clean old and dying red blood cells like the spleen. Plasmodium gallinacean does not harm ducks anyway, and even more amazingly they seem to reproduce and to function like some normal flora in ducks.
Erythrocytes are critical to transporting oxygen, so as long as Plasmodium stays out of them (as an intracellular parasite), it may serve beneficially as an immune stimulator/modulator in the RES. The ancestors to Plasmodium in the beginning (Genesis 1 and 2) probably were not intended for erythrocytes. They primarily inflict the greatest damage to the organism as a whole once they become a destructive intracellular parasite in the blood. The cyclic bouts of fever and chills in malaria are caused by massive red blood cell lysis.
According to the Microbial Deprivation Hypothesis (Roberts, Janovy, and Nadler, p. 38) the proper development of the animal immune system depends on continuous exposure to a variety of antigens, among them are bacteria and parasites. Studies have found an inverse relationship between some autoimmune diseases and parasitic diseases (i.e. the less exposure to parasite antigens, the greater risk of having an autoimmune disease). This is evidently resulting from a variety of mechanisms that affect T-cell activation, cytokine levels, Toll Like Receptor (TLR) signaling, dendritic cell function and other aspects of the immune response. Diseases (such as autoimmune diseases) involved in these studies include inflammatory bowel disease, multiple sclerosis, lupus, and Type 1 diabetes, etc. It would not be surprising if Plasmodium gallinacean was originally designed to stimulate antigens in wild animals for their proper immune system maturity the way we see in birds today, like ducks.
In mammals, Plasmodium may have stimulated lysosomal granules that aid in controlling immune function. Today we know that Plasmodium acts like neutrophils that restrain bacteria (Roberts, Janovy, and Nadler 2013, p. 147) by patroling the bloodstream to phagocytize pathogens.
Plasmodium can evade vertebrate immune host response because they are hidden inside red blood cells and they have an amazing ability to rapidly adjust to host physiological changes: compelling evidence that they were designed to do so. Indeed, people who are Duffy-blood-group negative are completely resistant to Plasmodium vivax (Fig. 9). This suggests that early in human ancestry, there may have been complete tolerance or at least resistance to malaria. Like birds, it may be that a more robust immune response (in mammals) is made to genuine microbial threats.
Plasmodium Diversity and “Descent”
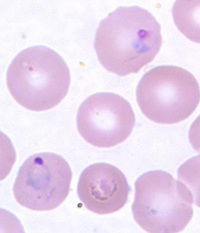
Fig. 9. Plasmodium vivax photo, CDC
Fig. 10. Plasmodium malariae (rosette formation) photo, CDC; Wikimedia Commons
There are over 250 species of Plasmodium known. They affect some reptiles, birds, mammals, primates and humans. Some of the most studied are those that infect birds, rodents, monkeys, chimpanzees, and humans. The intervals between successive attacks of chills and fever in infections depend on which species of malaria one is infected with. In human history (or, since the Fall) as many as 11 different species have infected people. Today, man can be infected with five different species of malaria: Plasmodium vivax, P. ovale, P. knowlesi, P. malariae, and P. falciparum. In the early history of the United States, the relatively mild P. vivax, or benign malaria, was the most common. By contrast the deadliest species today is malignant malaria, P. falciparum.
Although zoologists differ in their interpretation of Plasmodium “evolution,” most malariologists consider Plasmodium falciparum to have descended from species that infected a chimpanzee or gorilla and the other four Plasmodium species from old world monkeys (the Catarrhini). Of the three major species, P. malariae (Fig. 10) is considered to have been the first to infect humans, then P. vivax, and then P. falciparum. P. ovale is considered a variant of P. malariae and P. knowlesi has only recently “jumped” from monkeys to humans. Plasmodium falciparum is thought to have originated from Plasmodium reichenowi as recently as 6,000–10,000 years ago according to evolutionary biologists based upon genetic analysis and biogeography.
As mentioned, P. falciparum is by far the greatest threat to humans. It can infect up to 80% of its victims’ blood (approximately 24 trillion cells), forty times more than its cousin P. vivax. Its ring stage may have as many as 3 trophozoites per red blood cell; whereas P. vivax has only one trophozoite. P. falciparum accounts for 91% of malaria cases worldwide (Kalanon and McFadden 2010).
It is clear that Plasmodium shows signs of “intelligent design” having a complexity of both cell structure and life cycle. It has an amazing ability to adapt to various microhabitats. For example, it shows remarkable, even cunning, ways to evade host defenses in drastically different environments, such as mosquito hemolymph8 and human blood. Merozoites can freely move in the liver and avoid phagocytosis by Kupffer cells and remain (as trophozoites) in red blood cells as an immunoprivileged cell.
Mutualism and Creation Worldview of Parasites
Parasite origin will continue to challenge creation zoologists as a theoretical explanation is determined. What we do know is that God did not create after the first week of creation because Exodus 20:11 says that He finished His work of creation on the 6th day. If this is correct then the creatures that we call parasites today were beneficial, neutral, or free-living in their original form. A majority of parasites easily enter the host and have an amazing survival rate. Perhaps most creatures we call parasites were in a symbiotic relationship with their host at the “very good” original state. After the Fall and subsequent curse, degeneration, modification, mutation, adaptation, overgrowth, and displacement occurred. For example, there was a loss of genetic information and degeneration of cell structures.
One may wonder, in regard to intelligent design, why would a benevolent Creator make such creature that causes such misery? The answer is the original structure and life cycle were likely of algal origin. The proto-Plasmodium kind was likely designed in the manner of corals with dinoflagellates—abundant and helpful to the entire ecosystem. As in all theoretical scientific endeavors, research continues in this difficult area of parasite origin.
Beneficial symbiosis was most likely the norm in God’s Creation. There was a biomatrix—an interwoven organization (i.e. organosubstrate, Francis 2003)—throughout the entire earth. Parasitism is a secondary state in nature. The complexity of life cycles and “ease” of adaptation to other hosts points to malaria cargo as being symbiotic from the beginning. All humans and animals (and animal-like protists) were dependent upon plants, not blood or flesh for food in the original Creation (Genesis 1:29–30). We know that there is life in the blood, and in this broken/fallen world we suggest malaria (and any other disease) had its genesis after the Edenic Curse of Genesis 3.
Summary and Conclusions
The evolution model is one of upward, onward progression including serial endosymbiosis that has one cell kind ensconced within another. Plasmodium entered the life cycle evolving in the mosquito, then vertebrates (including mammals), and ultimately man. The creation model offers a better explanation for the observed Plasmodium “kind.” It predicts that all microbial life was created as kinds, subject to limited change or variation, including decay in its genome and structure. The Plasmodium “kind” was fully developed in form and life cycle, functional, and genetically isolated as a kind. No evidence of evolution from one kind of organism to another exists. Further, the mere probabilities against the natural evolution of the Plasmodium kind favor the supernatural origin of the Plasmodium kind, and laboratory evidence of Crithidia easily converting from blood- to plant-based food supports the concept of limited change within each kind.
In the “very good” pre-Fall world there was true ecological harmony with an intimate association of individuals of different species (symbiosis). Creation microbiologists are currently investigating the role of single-celled eukaryotic creatures in the organosubstrate model. While the controlling factors that direct invasiveness of Plasmodium are not well understood, the progression from originally free-living single-celled eukaryotes (neutral or beneficial) toward a pathogenic condition after the Fall could have occurred through a number of mechanisms leading to a parasitic condition.
References
Enserink, M. 2013. To stop malaria, infect the mosquitoes. Science Now (9 May 2013), http://www.sciencemag.org/news/2013/05/stop-malaria-infect-mosquitoes.
Francis, J. W. 2003. The organosubstrate of life: a creationist perspective of microbes and viruses. In Proceedings of the Fifth International Conference on Creationism, ed. R. L. Ivey, pp. 434–444. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511; doi:10.1038/nature01097 in Plasmodium genomics special issue, 3 October 2002.
Gillen, A. L. 2013. Parasitology Lab Manual, 2nd edition. Kearney, Nebraska: RL Simonson Studios.
Gillen, A. L. 2007. The Genesis of Germs: Disease and the Coming Plagues in a Fallen World. Green Forest, Arkansas: Master Books.
Kalanon, M. and G. McFadden. 2010. Malaria, Plasmodium falciparum, and its apicoplast. Biochemical Society transactions 38(3):775–82. doi: 10.1042/BST0380775. Retrieved from liberty.illiad.oclc.org.
Marquardt, W. C., R. S. Demaree, and R. B. Grieve. 2000. Parasitology and Vector Biology, 2nd edition. San Diego, California: Harcourt Academic Press.
Perry, A. 2011. Lifeblood. New York: PublicAffairs.
Roberts, L. S., J. Janovy, Jr., and S. Nadler. 2013. Schmidt and Roberts’ Foundations of Parasitology, 9th edition. Boston, Massachusetts: WCB McGraw-Hill.
Shah, S. 2011. The Fever: How Malaria Has Ruled Humankind for 500,000 Years (reprint edition). New York: Picador.
Short, A. R. 1955. The Bible and Modern Medicine. London: The Paternoster Press.
Smith, D. 2013. Steal my sunshine. The Scientist 27:35–40.
Smyth, J. D. 1976. Introduction to Animal Parasitology. London: Hodder and Stoughton.
Spielman, A. and M. D’Antonio, 2001. Mosquito: A Natural History of Our Most Persistent and Deadly Foe, 1st edition. New York: Hyperion.
Tortora, G. J., B. R. Funke, and C. L. Case. 2013. Microbiology, An Introduction,11th edition. San Francisco, California: Pearson Benjamin/Cummings Pub. Co.
World Health Organization. 2012. World Malaria Report 2012 FACT SHEET (published 17 December 2012), www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_factsheet.pdf.
World Health Organization. 2013. Malaria fact sheet. Retrieved from www.who.int/mediacentre/factsheets/fs094/en/index.html.
Footnotes
- The dates given in the article are common ones reported in the secular literature. In reality, we do not know the exact dates. In general, those dates given in thousands of years by secular authors may be slightly exaggerated, but are not far from a biblical time frame. The main point of these dates given (in thousands of years) is to point out that malaria is an ancient disease, afflicting many during the time that the book of Genesis was written. Of course, this is very different from the evolutionary model for Plasmodium’s origin dating millions of years ago.
- Interwoven together: this implies a mutualistic relationship between two distinct organisms, not a mosaic of one.
- Apicoplasts can only be seen in two stages (sporozoite and merozoite) of the mosquito life cycle through an electron microscope. They cause parasite death making them a promising target for future chemotherapy. Because the apicoplasts degenerated from chloroplasts (as will be discussed below), they have metabolic processes similar to that of bacteria and algae.
- Chloroplasts are the food producers of the plant cells. They are in charge of photosynthesis. They use carbon dioxide and free oxygen to photosynthesize. They use the assistance of ATP and NADPH. They contain green pigment, but they are not all green in color. They are members of the part of the cell called plastids.
- Leghemoglobin is a “blood-like” protein found in the roots of leguminous plants, specifically their nodules that contain nitrogen-fixing bacteria (Rhizobium). Leghemoglobin is produced by plant roots infected by this bacteria, as part of the designed mutualistic (symbiotic) interaction between plant and bacterium. Any roots uninfected with Rhizobium do not synthesize leghemoglobin. This protein has close chemical and structural similarities to hemoglobin, and, like hemoglobin, is red in color. Leghemoglobin is a product of both plant and bacteria. The protein is produced by the plant genes and the heme group (containing iron) is produced by the bacterium. Prior to the Fall and Edenic Curse, protozoans and arthropods utilized this plant-based protein instead of hemoglobin. Mosquito and their cargo now utilize blood for egg production, but they may have once briefly been able to have their nutritional needs met by plant protein.
- Zoonosis is an infectious or parasitic disease that is transmitted from an animal (sometime by a vector).
- The reticuloendothelial system, formerly the monocytic macrophage system, is part of the immune system that clears abnormal and old cells.
- Hemolymph is a fluid in the mosquito circulatory system. It is analogous to the blood, fluids and cells making up the vertebrate (birds, mammals, man) cardiovascular system. Hemolymph fills the interior of the mosquito’s body and surrounds all cells. Hemolymph is composed of water, salts, and organic compounds such as carbohydrates, proteins, and lipids. The immune system resides in the hemolymph.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


