
Chapter 11
Carbon Dating: Questions Answered
The most well-known of all the radiometric dating methods is radiocarbon dating (or carbon dating). Although many people think radiocarbon is used to date rocks, it is limited to dating things that contain carbon and were once alive (fossils).
How Radiocarbon Forms
Radiocarbon (carbon-14 or 14C) forms continually today in the earth’s upper atmosphere. And as far as we know, it has been forming in the earth’s upper atmosphere at least since the Fall, after the atmosphere was made back on Day Two of creation week (part of the expanse, or firmament, described in Genesis 1:6–8).
So how does radiocarbon form? Cosmic rays from outer space are continually bombarding the upper atmosphere of the earth, producing fast-moving neutrons (sub-atomic particles carrying no electric charge) (figure 1).1 These fast-moving neutrons collide with nitrogen-14 atoms, the most abundant element in the upper atmosphere, converting them into radiocarbon (carbon-14) atoms.
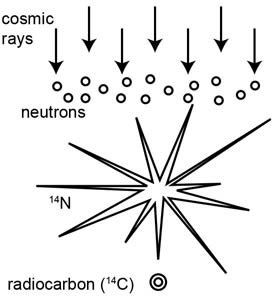
Figure 1. The formation of radiocarbon (14C or carbon-14) in the earth’s upper atmosphere due to the influx of cosmic rays from outer space.
Since the atmosphere is composed of about 78 percent nitrogen,2 a lot of radiocarbon atoms are produced—in total about 16.5 lbs. (7.5 kg) per year. These rapidly combine with oxygen atoms (the second most abundant element in the atmosphere, at 21 percent) to form carbon dioxide (CO2).
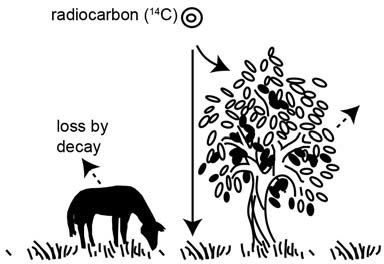
Figure 2. Radiocarbon (14C or carbon-14) atoms combine with oxygen atoms in the atmosphere to form carbon dioxide (CO2) that circulates into the biosphere. Radiocarbon is thus incorporated into plants by photosynthesis and into the animals that eat the plants. Continued photosynthesis and feeding replaces the 14C atoms lost from the plants and animals by decay back to 14N (nitrogen-14).
This carbon dioxide, now radioactive with carbon-14, is otherwise chemically indistinguishable from the normal carbon dioxide in the atmosphere, which is slightly lighter because it contains normal carbon-12. Radioactive and non-radioactive carbon dioxide mix throughout the atmosphere, and dissolve in the oceans. Through photosynthesis carbon dioxide enters plants and algae, bringing radiocarbon into the food chain. Radiocarbon then enters animals as they consume the plants (figure 2). So even we humans are radioactive because of trace amounts of radiocarbon in our bodies.
Carbon Dating: Determining the Rate of Radiocarbon Decay
After radiocarbon forms, the nuclei of the carbon-14 atoms are unstable, so over time they progressively decay back to nuclei of stable nitrogen-14.3 A neutron breaks down to a proton and an electron, and the electron is ejected. This process is called beta decay. The ejected electrons are called beta particles and make up what is called beta radiation.
Not all radiocarbon atoms decay at the same time. Different carbon-14 atoms revert to nitrogen-14 at different times, which explains why radioactive decay is considered a random process. To measure the rate of decay, a suitable detector records the number of beta particles ejected from a measured quantity of carbon over a period of time, say a month (for illustration purposes). Since each beta particle represents one decayed carbon-14 atom, we know how many carbon-14 atoms decayed during that month.
Chemists have already determined how many atoms are in a given mass of each element, such as carbon.4 So if we weigh a lump of carbon, we can calculate how many carbon atoms are in it. If we know what fraction of the carbon atoms are radioactive, we can also calculate how many radiocarbon atoms are in the lump. Knowing the number of atoms that decayed in our sample over a month, we can calculate the radiocarbon decay rate.
The standard way of expressing the decay rate is called the half-life.5 It’s defined as the time it takes half a given quantity of a radioactive element to decay. So if we started with 2 million atoms of carbon-14 in our measured quantity of carbon, then the half-life of radiocarbon will be the time it takes for half, or 1 million, of these atoms to decay. The radiocarbon half-life or decay rate has been determined at 5,730 years.
Using Radiocarbon for Dating
Next comes the question of how scientists use this knowledge to date things. If carbon-14 has formed at a constant rate for a very long time and continually mixed into the biosphere, then the level of carbon-14 in the atmosphere should remain constant. If the level is constant, living plants and animals should also maintain a constant carbon-14 level in them. The reason is that, as long as the organism is alive, it replaces any carbon molecules that have decayed into nitrogen.
After plants and animals perish, however, they no longer replace molecules damaged by radioactive decay. Instead, the radiocarbon atoms in their bodies slowly decay away, so the ratio of carbon-14 atoms to regular carbon atoms will steadily decrease over time (figure 3).
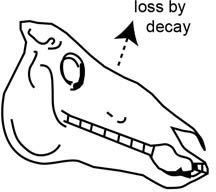
Figure 3. After the death of an animal it no longer eats and adds 14C to its body, so the 14C in it is steadily lost by decay back to 14N.
Let’s suppose we find a mammoth’s skull, and we want to date it to determine how long ago it lived. We can measure in the laboratory how many carbon-14 atoms are still in the skull. If we assume that the mammoth originally had the same number of carbon-14 atoms in its bones as living animals do today (estimated at one carbon-14 atom for every trillion carbon-12 atoms), then, because we also know the radiocarbon decay rate, we can calculate how long ago the mammoth died. It’s really quite that simple.
This dating method is also similar to the principle behind an hourglass (figure 4). The sand grains that originally filled the top bowl represent the carbon-14 atoms in the living mammoth just before it died. It’s assumed to be the same number of carbon-14 atoms as in elephants living today. With time, those sand grains fell to the bottom bowl, so the new number represents the carbon-14 atoms left in the mammoth skull when we found it. The difference in the number of sand grains represents the number of carbon-14 atoms that have decayed back to nitrogen-14 since the mammoth died. Because we have measured the rate at which the sand grains fall (the radiocarbon decay rate), we can then calculate how long it took those carbon-14 atoms to decay, which is how long ago the mammoth died.
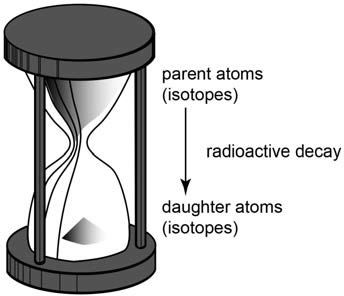
Figure 4. A simple hourglass clock. The sand grains in the top bowl fall to the bottom bowl to measure the passage of time. If all the sand grains are in the top bowl, then it takes exactly an hour for them all to fall. So if half the sand grains are in the top bowl and half in the bottom bowl, then 30 minutes has elapsed since the sand grains began falling. We can calibrate an hourglass clock by timing the falling sand grains against a mechanical or electronic clock. But there is no way of independently calibrating the radioactive clocks in rocks because no observers were present when the rocks formed and the clocks started.
That’s how the radiocarbon method works. And because the half-life of carbon-14 is just 5,730 years, radiocarbon dating of materials containing carbon yields dates of only thousands of years, not the dates over millions of years that conflict with the framework of earth history provided by the Bible, God’s eyewitness account of history.
So one would think that since the radiocarbon dating method works on organic (once-living) materials, then radiocarbon could be used to date fossils. After all, we should be able to estimate how long ago a creature lived based on how much radiocarbon is left in its body.
Why Isn’t Carbon Dating Used to Date Fossils?
The answer is a matter of basic physics. Radiocarbon (carbon-14) is a very unstable element that quickly changes into nitrogen. Half the original quantity of carbon-14 will decay back to the stable element nitrogen-14 after only 5,730 years. (This 5,730 year period is called the half-life of radiocarbon, figure 5).6 At this decay rate, hardly any carbon-14 atoms will remain after only 57,300 years (or ten half-lives).
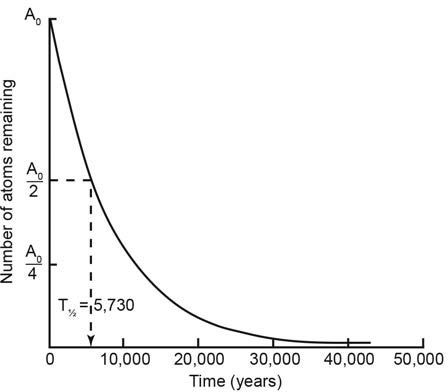
Figure 5. The decay of radiocarbon follows the exponential decay law, whereby the percentage decrease in the number of parent atoms per unit time is constant. After each half-life of 5,730 years, the number of parent radiocarbon atoms remaining is halved.
So if fossils are really millions of years old, as evolutionary scientists claim, no carbon-14 atoms would be left in them. Indeed, if all the atoms making up the entire earth were radiocarbon, then after only 1 million years absolutely no carbon-14 atoms should be left!
The Power of Radiocarbon Detection Technology
Most laboratories measure radiocarbon with a very sophisticated instrument called an accelerator mass spectrometer, or AMS. It is able to literally count carbon-14 atoms one at a time.7 This machine can theoretically detect one radioactive carbon-14 atom in 100 quadrillion regular carbon-12 atoms! However, there’s a catch! AMS instruments need to be checked occasionally to make sure they aren’t also “reading” any laboratory contamination, called background. So rock samples that should read zero are occasionally placed into these instruments to test their accuracy. What better samples to use than fossils, coals, and limestones, which are supposed to be millions of years old and should have no radiocarbon?
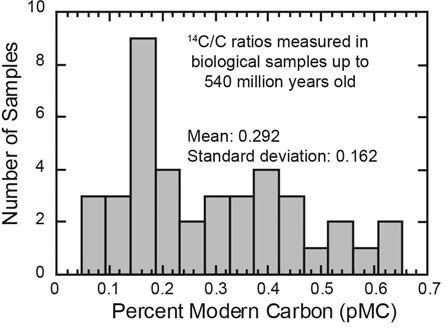
Figure 6. Distribution of 14C values in samples of organic carbon from biologically derived materials such as fossils, limestones, coals, oils, natural gas, and graphite, as reported in the scientific literature. All these samples are supposed to be millions of years old and should contain no detectable radiocarbon, according to the standard geological time scale.
Radiocarbon Found!
Imagine the surprise when every piece of “ancient” carbon tested has contained measurable quantities of radiocarbon!8 Fossils, coal, oil, natural gas, limestone, marble, and graphite from every Flood-related rock layer—and even some pre-Flood deposits—have all contained measurable quantities of radiocarbon (figure 6). All these results have been reported in the conventional scientific literature.
This finding is consistent with the belief that rocks are only thousands of years old, but the specialists who obtained these results have definitely not accepted this conclusion. It does not fit their presuppositions. To keep from concluding that the rocks are only thousands of years old, they claim that the radiocarbon must be due to contamination, either from the field or from the laboratory, or from both. However, when technicians meticulously clean the rocks with hot strong acids and other harsh pre-treatments to remove any possible contamination, these “ancient” organic (once-living) materials still contain measurable radiocarbon.
Since a blank sample holder in the AMS instrument predictably yields zero radiocarbon, these scientists should naturally conclude that the radiocarbon is “intrinsic” to the rocks. In other words, real radiocarbon is an integral part of the “ancient” organic materials. But these scientists’ presuppositions prevent them from reaching this conclusion.
Radiocarbon in Fossils Confirmed
For some years creation scientists have been doing their own investigations of radiocarbon in fossils. Pieces of fossilized wood in Oligocene, Eocene, Cretaceous, Jurassic, Triassic, and Permian rock layers supposedly 32 to 250 million years old all contain measurable radiocarbon, equivalent to “ages” of 20,700 to 44,700 years.9 (Creation geologists believe that with careful recalibration, even these extremely “young” ages would be less than 10,000 years.)
Similarly, carefully sampled pieces of coal from 10 US coal beds, ranging from Eocene to Pennsylvanian and supposedly 40 to 320 million years old, all contained similar radiocarbon levels equivalent to “ages” of 48,000 to 50,000 years.10 Even fossilized ammonite shells found alongside fossilized wood in a Cretaceous layer, supposedly 112 to 120 million years old, contained measurable radiocarbon equivalent to “ages” of 36,400 to 48,710 years.11
Radiocarbon Is Even in Diamonds
Just as intriguing is the discovery of measurable radiocarbon in diamonds. Creationist and evolutionary geologists agree that diamonds are formed more than 100 miles (160 km) down, deep within the earth’s upper mantle, and do not consist of organic carbon from living things. Explosive volcanoes brought them to the earth’s surface very rapidly in “pipes.” As the hardest known natural substance, these diamonds are extremely resistant to chemical corrosion and external contamination. Also, the tight bonding in their crystals would have prevented any carbon-14 in the atmosphere from replacing any regular carbon atoms in the diamonds.
Yet diamonds have been tested and shown to contain radiocarbon equivalent to an “age” of 55,000 years.12 These results have been confirmed by other investigators.13 And calculations have shown that any radiation from trace uranium in the earth near the diamonds would have been totally incapable of producing from any nitrogen in the diamonds these measured levels of in situ carbon-14.14 So even though these diamonds are conventionally regarded by evolutionary geologists as up to billions of years old, this radiocarbon has to be intrinsic to them. This carbon-14 would have been implanted in them when they were formed deep inside the earth, and it could not have come from the earth’s atmosphere. This is not a problem for creationist scientists, but it is a serious problem for evolutionists.
The Radiocarbon “Puzzle”
Evolutionary radiocarbon scientists have still not conceded that fossils, coals, and diamonds are only thousands of years old. Their uniformitarian (slow-and-gradual) interpretation requires that the earth’s rocks be millions or billions of years old. They still maintain that the carbon-14 is “machine background” contaminating all these tested samples. Among their proposed explanations is that the AMS instruments do not properly reset themselves between sample analyses. But if this were true, why does the instrument find zero atoms when no sample is in it?
It should be noted that radiocarbon “ages” of up to 50,000 years don’t match the biblical time frame either. The Flood cataclysm was only about 4,350 years ago. However, these young radiocarbon “ages” are far more in accord with the Bible’s account than the uniformitarian time scale. The discovery that diamonds have 55,000-year radiocarbon “ages” may help us unravel this mystery.
However, it would be extremely helpful if it were possible to systematically recalibrate radiocarbon “ages.” Once radiocarbon is interpreted properly, it should help creationists date archeological remains from post-Flood human history, showing how they fit within the Bible’s chronology.
Assumptions Change Estimates of Age
To solve this puzzle it is necessary to review the assumptions on which radiocarbon dating is based. These include15
- The production rate of carbon-14 has always been the same in the past as now.
- The atmosphere has had the same carbon-14 concentration in the past as now.
- The biosphere (the places on earth where organisms live) has always had the same overall carbon-14 concentration as the atmosphere, due to the rapid transfer of carbon-14 atoms from the atmosphere to the biosphere.
None of these assumptions is strictly correct, beyond a rough first approximation. Indeed, scientists have now determined that the concentration of carbon-14 in the atmosphere varies considerably according to latitude. They have also determined several geophysical causes for past and present fluctuations in carbon-14 production in the atmosphere.16
Specifically, we know that carbon-14 has varied in the past due to a stronger magnetic field on the earth and changing cycles in sunspot activity. So when objects of known historical dates are dated using radiocarbon dating, we find that carbon-14 dates are accurate back to only about 400 B.C.
The conventional scientific community is ignoring at least two factors crucial to recalibrating radiocarbon (so that it accounts for major changes in the biosphere and atmosphere that likely resulted from the Flood): (1) the earth’s magnetic field has been progressively stronger going back into the past, and (2) the Flood destroyed and buried a huge amount of carbon from the pre-Flood biosphere.
The Effect of a Past Stronger Magnetic Field
The evidence for the earth having a progressively stronger magnetic field going back into the past is based on reliable historical measurements17 and “fossil” magnetism trapped in ancient pottery.18,19
A stronger magnetic field is significant because the magnetic field partly shields the earth from the influx of cosmic rays,20 which change nitrogen atoms into radioactive carbon-14 atoms. So a stronger magnetic field in the past would have reduced the influx of cosmic rays. This in turn would have reduced the amount of radiocarbon produced in the atmosphere. If this were the case, the biosphere in the past would have had a lower carbon-14 concentration than it does today.
The best estimates indicate that the earth’s magnetic field was twice as strong only 1,400 years ago and possibly four times as strong 2,800 years ago. If this is true, the earth’s magnetic field would have been much stronger at the time of the Flood, and the carbon-14 levels in the biosphere would have been significantly smaller.
So if you mistakenly assume that the radiocarbon levels in the atmosphere and biosphere have always been the same as they are today, you would erroneously estimate much older dates for early human artifacts, such as post-Babel wooden statuettes in Egypt. And that is exactly what conventional archaeology has done.
The Effect of More Carbon in the Pre-Flood Biosphere
An even more dramatic effect on the earth’s carbon-14 inventory would be the destruction and burial of all the carbon in the whole biosphere at the time of the Flood. Based on the enormous size of today’s coal beds, oil, oil shale, and natural gas deposits, and all the fossils in limestones, shales, and sandstones, a huge quantity of plants and animals must have been alive when the Flood struck. It is conservatively estimated that the amount of carbon in the pre-Flood biosphere may have been many times greater than the amount of carbon in today’s biosphere.21
We cannot yet know for certain how much radiocarbon (carbon-14) was in this pre-Flood carbon (a mixture of normal carbon-12 and carbon-14). Yet if the earth’s atmosphere started to produce carbon-14 at the Fall, then many radiocarbon atoms could have been in the pre-Flood biosphere by the time of the Flood, about 1,650 years after creation. However, if there was a whole lot more normal carbon (carbon-12 or 12C) in the pre-Flood biosphere, then the proportion of 14C to 12C would have been very much less than the proportion in today’s biosphere.
So when scientists fail to account for so many more plants and animals in the lush pre-Flood biosphere and wrongly assume that plants buried in coal beds had the same proportion of carbon-14 as plants do today, then their radiocarbon dating would yield “ages” very much higher than the true Flood age of about 4,350 years.
A Prediction Fulfilled
Now if this model of the earth’s past radiocarbon inventory is correct, then a logical prediction follows. Since all pre-Flood plants would have had the same low radiocarbon levels when they were buried, and they all formed into coal beds during that single Flood year, then those coal beds should all have the same low radiocarbon content.
They do! Samples from coal beds around the United States, ranging from Eocene to Pennsylvanian deposits, supposedly 40 to 320 million years old, all contain the same low radiocarbon levels equivalent to “ages” of 48,000 to 50,000 years.22 This makes sense only if these coal beds were all formed out of pre-Flood plants during the yearlong Flood, about 4,350 years ago. Carbon-14 dates of the same value are expected in creation theory and contrary to the expectations in conventional old-earth theory.
The “Puzzle” Is Being Solved
So the radiocarbon “puzzle” can be solved, but only in the biblical framework for earth history. Research is therefore underway to find a means of recalibrating the radiocarbon “clock” to properly account for the Flood and its impact on dates for the post-Flood period to the present.
For example, conventional radiocarbon dating gives an age of “48,000 years” for a coal bed deposited during the Flood, about 4,350 years ago. This could be explained if the 14C/12C ratio at the time of the Flood was only 1/200th the ratio of the present world. If scientists assume the ratio is 200 times greater than it really was, then their radiocarbon age estimate would be exaggerated by 43,650 years.23
In reality, calculations (described above) have led to estimates that the pre-Flood biosphere may have had more than 100 times the carbon-12 as the present earth. Using this information, we may be able to calculate how much carbon-14 was actually on the early earth at the Flood. This, in turn, would allow us to develop a proper interpretation of all carbon-14 dates.
Once the research is completed, one exciting benefit is that it should be possible to begin more accurately dating any archaeological artifact within the true chronology of history found in God’s Word.
How Do We Know the Bible Is True? Volume 2
Join a team of biblical scholars and Christian apologists present answers to twenty more relevant debates. Be prepared to give a reason for your faith in God!
Read OnlineOriginally published as “Radiocarbon Dating?”
Footnotes
- Sheridan Bowman, Interpreting the Past: Radiocarbon Dating (London: British Museum Publications, 1990).
- Steven S. Zumdahl, Chemical Principles, second edition (Lexington, MA: D.C. Heath and Company, 1995), p.171.
- Alan P. Dickin, Radiogenic Isotope Geology, second edition (Cambridge, UK: Cambridge University Press, 2005), p. 383–398.
- Zumdahl, Chemical Principles, p. 55. For radiocarbon this number is ~6.022 X 1023 atoms per 14 grams of carbon-14.
- Gunter Faure and Teresa M. Mensing, Isotopes: Principles and Applications, third edition (Hoboken, NJ: John Wiley & Sons, 2005), p. 614–625.
- Bowman, Sheridan, Interpreting the Past: Radiocarbon Dating, University of California Press, 1990), p. 614–625.
- Dickin, Radiogenic Isotope Geology, p. 383–398.
- Robert L. Whitelaw, “Time, Life, and History in the Light of 15,000 Radiocarbon Dates,” Creation Research Society Quarterly 7 no.1 (1970): p. 56–71; Paul Giem, “Carbon-14 Content of Fossil Carbon,” Origins 51 (2001): p. 6–30.
- Dr. Andrew A. Snelling, “Radioactive ‘Dating’ in Conflict! Fossil Wood in ‘Ancient’ Lava Flow Yields Radiocarbon,” Creation 20 no.1 (1997): p. 24–27; Dr. Andrew A. Snelling, “Stumping Old-age Dogma: Radiocarbon in ‘Ancient’ Fossil Tree Stump Casts Doubt on Traditional Rock/Fossil Dating,” Creation 20 no.4 (1998): p. 48–51; Dr. Andrew A. Snelling, “Dating Dilemma: Fossil Wood in ‘Ancient’ Sandstone,” Creation 21 no.3 (1999): p. 39–41; Dr. Andrew A. Snelling, “Geological Conflict: Young Radiocarbon Date for ‘Ancient’ Fossil Wood Challenges Fossil Dating,” Creation 22 no.2 (2000): p. 44–47; Dr. Andrew A. Snelling, “Conflicting ‘Ages’ of Tertiary Basalt and Contained Fossilised Wood, Crinum, Central Queensland, Australia,” CEN Technical Journal 14 no.2 (2000): p. 99–122; Dr. Andrew A. Snelling, “Radiocarbon in ‘Ancient’ Fossil Wood,” Impact #415, Acts & Facts, Institute for Creation Research (January 2008): p. 10–13; Dr. Andrew A. Snelling, “Radiocarbon Ages for Fossil Ammonites and Wood in Cretaceous Strata Near Redding, California,” Answers Research Journal 1 (2008): p. 123–144.
- John R. Baumgardner, Dr. Andrew A. Snelling, D. Russell Humphreys, and Steven A. Austin, Proceedings of the Fifth International Conference on Creationism, “Measurable 14C in Fossilized Organic Materials: Confirming the Young Earth Creation-Flood Model,” Robert L. Ivey Jr., editor (Pittsburgh, PA: Creation Science Fellowship, 2003), p. 127–147.
- Dr. Andrew A. Snelling, “Radiocarbon Ages for Fossil Ammonites and Wood in Cretaceous Strata near Redding, California,” Answers Research Journal 1 (2008): p. 123–144.
- John R. Baumgardner, Radioisotopes and the Age of the Earth: Results of a Young Earth Creationist Research Initiative, “14C Evidence for a Recent Global Flood and a Young Earth,” Larry Vardiman, Dr. Andrew A. Snelling, and Eugene F. Chaffin, editors, (El Cajon, CA: Institute for Creation Research, and Chino Valley, AZ: Creation Research Society, 2005), p. 587–630; Don B. DeYoung, Thousands . . . Not Billions: Challenging an Icon of Evolution, Questioning the Age of the Earth (Green Forest, AR: Master Books, 2005), p. 45–62.
- R. Ervin Taylor and John Southon, “Use of Natural Diamonds to Monitor 14C AMS Instrument Backgrounds,” Nuclear Instruments and Methods in Physics Research B 259 (2007): p. 282–287.
- Baumgardner, “14C Evidence for a Recent Global Flood and a Young Earth,” p. 614–616.
- Sheridan Bowman, Interpreting the Past: Radiocarbon Dating (London: British Museum Publications, 1990), p. 14.
- Ibid., p. 16–30; Gunter Faure and Teresa M. Mensing, Isotopes: Principles and Applications, third edition (Hoboken, NJ: John Wiley & Sons, 2005), p. 614–625; Alan P. Dickin, Radiogenic Isotope Geology, second edition (Cambridge, UK: Cambridge University Press, 2005), p. 383–398.
- Thomas G. Barnes, “Decay of the Earth’s Magnetic Field and the Geochronological Implications,” Creation Research Society Quarterly 8 no.1 (1971): p. 24–29; Thomas G. Barnes, “Electromagnetics of the Earth’s Field and Evaluation of Electric Conductivity, Current and Joule Peaking in the Earth’s Core,” Creation Research Society Quarterly 9 no. 4 (1973): p. 222–230; D. Russell Humphreys, “The Creation of the Earth’s Magnetic Field,” Creation Research Society Quarterly 20 no. 1 (1983): p. 89–90;
- D. Russell Humphreys Proceedings of the First International Conference on Creationism, volume II, “Reversals of the Earth’s Magnetic Field During the Genesis Flood,” Robert E. Walsh, Christopher L. Brooks, and Richard S. Crowell, editors, (Pittsburgh, PA: Creation Science Fellowship,1986), p. 113–123.
R.T. Merrill and N.W. McElhinney, The Earth’s Magnetic Field (London: Academic Press, 1983); Humphreys, “Reversals of the Earth’s Magnetic Field During the Genesis Flood,” p. 113–123. What is “fossil” magnetism? The clay used to make pottery contains mineral grains that are slightly magnetic. When the clay is baked, the grains’ magnetic field imprint at the time is “locked in” or fossilized.
The strength of the magnetic field was not affected by field reversals. The sun also regularly experiences field reversals without loss of strength in the magnetic field. Robert E. Walsh and Christopher L. Brooks, editors, Proceedings of the Second International Conference on Creationism, volume 2, “Physical Mechanism for Reversal of the Earth’s Magnetic Field During the Flood,” by D. Russell Humphreys (Pittsburgh, PA: Creation Science Fellowship, 1990), p. 129–142.
- R. Ervin Taylor, Austin Long, and Renee S. Kra, editors, Radiocarbon after Four Decades: An Interdisciplinary Perspective, “Radiocarbon Fluctuations and the Geomagnetic Field,” by Robert S. Sternberg (New York: Springer-Verlag, 1992), p. 93–116.
- Robert H. Brown, “The Interpretation of C-14 Dates,” Origins 6 (1979): p. 30–44; Glenn R. Morton, “The Carbon Problem,” Creation Research Society Quarterly 20 no. 4 (1984): p. 212–219; H.W. Scharpenseel and Peter Becker-Heidmann, “Twenty-five Years of Radiocarbon Dating Soils: Paradigm of Erring and Learning,” Radiocarbon 34 (1992): p. 541–549; Baumgardner, Snelling, Humphreys, and Austin, “Measurable 14C in Fossilized Organic Materials: Confirming the Young Earth Creation-Flood Model,” p. 127–147.
- Baumgardner, “14C Evidence for a Recent Global Flood and a Young Earth,” p. 614–616; DeYoung, Thousands . . . Not Billions: Challenging an Icon of Evolution, Questioning the Age of the Earth, p. 45–62.
- These numbers are calculated in terms of half-lives, discussed earlier. If the modern ratio is 200 times greater than the ratio at the Flood, the error ends up being 7.618 carbon-14 half-lives, or 43,650 years!
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


