
Measurable 14C in Fossilized Organic Materials: Confirming the Young Earth Creation-Flood Model
Abstract
Given the short 14C half-life of 5730 years, organic materials purportedly older than 250,000 years, corresponding to 43.6 half-lives, should contain absolutely no detectable 14C. (One gram of modern carbon contains about 6 × 1010 14C atoms, and 43.6 half-lives should reduce that number by a factor of 7.3 × 10-14.) An astonishing discovery made over the past 20 years is that, almost without exception, when tested by highly sensitive accelerator mass spectrometer (AMS) methods, organic samples from every portion of the Phanerozoic record show detectable amounts of 14C! 14C/C ratios from all but the youngest Phanerozoic samples appear to be clustered in the range 0.1–0.5 pmc (percent modern carbon), regardless of geological “age.” A straightforward conclusion that can be drawn from these observations is that all but the very youngest Phanerozoic organic material was buried contemporaneously much less than 250,000 years ago. This is consistent with the biblical account of a global Flood that destroyed most of the air-breathing life on the planet in a single brief cataclysm only a few thousand years ago.
Keywords: radiocarbon, AMS 14C analysis, 14C dead, 14C background, 14C contamination, uniformitarianism, young earth, Genesis Flood
This paper was originally published in the Proceedings of the Fifth International Conference on Creationism, pp. 127–142 (2003) and is reproduced here with the permission of the Creation Science Fellowship of Pittsburgh (www.csfpittsburgh. org).
Introduction
Giem1 reviewed the literature and tabulated about 70 reported AMS measurements of 14C in organic materials from the geologic record that, according to the conventional geologic time-scale, should be 14C “dead.” The surprising result is that organic samples from every portion of the Phanerozoic record show detectable amounts of 14C. For the measurements considered most reliable, the 14C/C ratios appear to fall in the range 0.1–0.5% of the modern 14C/C ratio (percent modern carbon, or pmc). Giem demonstrates instrument error can be eliminated as an explanation on experimental grounds. He shows contamination of the 14C-bearing fossil material in situ is unlikely but theoretically possible and is a testable hypothesis, while contamination during sample preparation is a genuine problem but largely solved by two decades of improvement in laboratory procedures. He concludes the 14C detected in these samples most likely is from the organisms from which the samples are derived. Moreover, because most fossil carbon seems to have roughly the same 14C/C ratio, Giem deems it plausible that all these organisms resided on earth at the same time.
Anomalous 14C in fossil material actually has been reported from the earliest days of radiocarbon dating. Whitelaw,2 for example, surveyed all the dates reported in the journal Radiocarbon up to 1970, and he commented that for all of the over 15,000 specimens reported, “All such matter is found datable within 50,000 years as published.” The specimens included coal, oil, natural gas, and other allegedly ancient material. The reason these anomalies were not taken seriously is because the older beta-decay counting technique had difficulty distinguishing genuine low levels of 14C in the samples from background counts due to cosmic rays. The AMS method, besides its inherently greater sensitivity, does not have this complication of spurious counts due to cosmic rays. In retrospect, it is likely that many of the beta-counting analyses were indeed truly detecting intrinsic 14C.
Measurable 14C in pre-Flood organic materials fossilized in Flood strata therefore appears to represent a powerful and testable confirmation of the young earth Creation-Flood model. It was on this basis that Snelling3,4,5,6,7 analyzed the 14C content of fossilized wood conventionally regarded as 14C “dead” because it was derived from Tertiary, Mesozoic, and upper Paleozoic strata having conventional radioisotope ages of 40 to 250 million years. All samples were analyzed using AMS technology by a reputable commercial laboratory with some duplicate samples also tested by a specialist laboratory in a major research institute. Measurable 14C was obtained in all cases. Values ranged from 7.58±1.11 pmc for a lower Jurassic sample to 0.38±0.04 pmc for a middle Tertiary sample (corresponding to 14C “ages” of 20,700±1200 to 44,700±950 years BP, respectively). The δ13C values for the samples clustered around –25‰, as expected for organic carbon in plants and wood. The 14C measured in these fossilized wood samples does not conform to a simple pattern, however, such as constant or decreasing with increasing depth in the geologic record (increasing conventional age). On the contrary, the middle Tertiary sample yielded the least 14C, while the Mesozoic and upper Paleozoic samples did not contain similar 14C levels as might be expected if these represent pre-Flood trees. The issue then of how uniformly the 14C may have been distributed in the pre-Flood world we concluded would likely be an important one. Therefore, our RATE team decided to undertake further 14C analyses on a new set of samples to address this issue as well as to confirm the remarkable 14C levels reported in the radiocarbon literature for Phanerozoic material.
14C Measured in Samples Conventionally Dated Older Than 100,000 Years
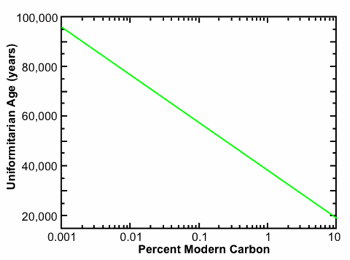
Fig. 1. Uniformitarian age as a function of 14C/C ratio in percent modern carbon. The uniformitarian approach for interpreting the 14C data assumes a constant 14C production rate and a constant biospheric carbon inventory extrapolated into the indefinite past. It does not account for the possibility of a recent global catastrophe that removed a large quantity of carbon from the biospheric inventory.
Giem8 compiled a long list of AMS measurements made on samples that, based on their conventional geological age, should be 14C “dead.” These measurements were performed in many different laboratories around the world and reported in the standard peer-reviewed literature, mostly in the journals Radiocarbon and Nuclear Instruments and Methods in Physics Research B. Despite the fact that the conventional uniformitarian age for these samples is well beyond 100,000 years (in most cases it is tens to hundreds of millions of years), it is helpful nonetheless to be able to translate 14C/C ratios into the equivalent uniformitarian 14C age under the standard uniformitarian assumptions of an approximately constant 14C production rate and an approximately constant biospheric carbon inventory, extrapolated into the indefinite past. This conversion is given by the simple formula, pmc = 100 × 2–t/5730, where t is the time in years. Applying this formula, one obtains values of 0.79 pmc for t = 40,000 years, 0.24 for t = 50,000 years, 0.070 pmc for 60,000 years, 0.011 pmc for 75,000 years, and .001 pmc for 95,000 years, as shown in graphical form in Fig. 1.
Table 1 contains most of Giem’s9 data plus data from some more recent papers. Included in the list are a number of samples from Precambrian, that is, what we consider non-organic pre-Flood settings. Most of the graphite samples with 14C/C values below 0.05 pmc are in this category.
We display the published AMS values of Table 1 in histogram format in Fig. 2. We have separated the source material into three categories, (1) those (mostly graphites) that are likely from Precambrian geological settings and unlikely to contain biological carbon, (2) those that are clearly of biological affinity, and (3) those (mostly marbles) whose biological connection is uncertain. We show categories (1) and (2) in Figs. 2(a) and 2(b), respectively, and ignore for these purposes samples in category (3). Some caution is in order with respect to the sort of comparison implicit in Table 1 and Fig. 2. In some cases the reported values have a “background” correction, typically on the order of 0.07 pmc, subtracted from the raw measured values, while in other cases such a correction has not been made. In most cases, the graphite results do not include such “background” corrections since they are usually intended themselves to serve as procedural blanks. Therefore, Fig. 2 is to be understood only as a low precision means for comparing these AMS results.
We draw several observations from this comparison, imprecise as it may be. First, the set of samples with biological affinity display a mean value significantly different from those without such affinity. In terms of the standard geological timescale, all these samples should be equally 14C dead. The samples with biological affinity display an unambiguously higher mean than those without such affinity, 0.29 versus 0.06 pmc. A second observation is that the variation in 14C content for the biological samples is large. Although a peak in the distribution occurs at about 0.2 pmc, the mean value is near 0.3 pmc with a standard deviation of 0.16 pmc.
Table 1. AMS measurements on samples conventionally deemed 14C “dead.”
| Item | 14C/C (pmc) (±1 S.D.) |
Material | Reference |
|---|---|---|---|
| 1 | 0.71±?* | Marble | Aerts-Bijma, Meijer,and van der Plicht10 |
| 2 | 0.65±0.04 | Shell | Beukens11 |
| 3 | 0.61±0.12 | Foraminifera | Arnold et al.12 |
| 4 | 0.60±0.04 | Commercial graphite | Schmidt, Balsley, and Leach13 |
| 5 | 0.58±0.09 | Foraminifera (Pyrgo murrhina) | Nadeau et al.14 |
| 6 | 0.54±0.04 | Calcite | Beukens15 |
| 7 | 0.52±0.20 | Shell (Spisula subtruncata) | Nadeau et al.16 |
| 8 | 0.52±0.04 | Whale bone | Jull et al.17 |
| 9 | 0.51±0.08 | Marble | Gulliksen and Thomsen18 |
| 10 | 0.5±0.1 | Wood, 60Ka | Gillespie and Hedges19 |
| 11 | 0.46±0.03 | Wood | Beukens20 |
| 12 | 0.46±0.03 | Wood | Vogel, Nelson, and Southon21 |
| 13 | 0.44±0.13 | Anthracite | Vogel, Nelson, and Southon22 |
| 14 | 0.42±0.03 | Anthracite | Grootes et al.23 |
| 15 | 0.401±0.084 | Foraminifera (untreated) | Schleicher et al.24 |
| 16 | 0.40±0.07 | Shell (Turitella communis) | Nadeau et al.25 |
| 17 | 0.383±0.045 | Wood (charred) | Snelling26 |
| 18 | 0.358±0.033 | Anthracite | Beukens27 |
| 19 | 0.35±0.03 | Shell (Varicorbula gibba) | Nadeau et al.28 |
| 20 | 0.342±0.037 | Wood | Beukens29 |
| 21 | 0.34±0.11 | Recycled graphite | Arnold et al.30 |
| 22 | 0.32±0.06 | Foraminifera | Gulliksen and Thomsen31 |
| 23 | 0.3±? | Coke | Terrasi et al.32 |
| 24 | 0.3±? | Coal | Schleicher et al.33 |
| 25 | 0.26±0.02 | Marble | Schmidt et al.34 |
| 26 | 0.2334±0.061 | Carbon powder | McNichol et al.35 |
| 27 | 0.23±0.04 | Foraminifera (mixed species avg.) | Nadeau et al.36 |
| 28 | 0.211±0.018 | Fossil wood | Beukens37 |
| 29 | 0.21±0.02 | Marble | Schmidt et al.38 |
| 30 | 0.21±0.06 | CO2 | Grootes et al.39 |
| 31 | 0.20–0.35* (range) | Anthracite | Aerts-Bijma et al.40 |
| 32 | 0.20±0.04 | Shell (Ostrea edulis) | Nadeau et al.41 |
| 33 | 0.20±0.04 | Shell (Pecten opercularis) | Nadeau et al.42 |
| 34 | 0.2±0.1* | Calcite | Donahue et al.43 |
| 35 | 0.198±0.060 | Carbon powder | McNichol et al.44 |
| 36 | 0.18±0.05 (range?) | Marble | Van der Borg et al.45 |
| 37 | 0.18±0.03 | Whale bone | Gulliksen and Thomsen46 |
| 38 | 0.18±0.03 | Calcite | Gulliksen and Thomsen47 |
| 39 | 0.18±0.01** | Anthracite | Nelson et al.48 |
| 40 | 0.18±? | Recycled graphite | Van der Borg et al.49 |
| 41 | 0.17±0.03 | Natural gas | Gulliksen and Thomsen50 |
| 42 | 0.166±0.008 | Foraminifera (treated) | Schleicher et al.51 |
| 43 | 0.162±? | Wood | Kirner et al.52 |
| 44 | 0.16±0.03 | Wood | Gulliksen and Thomsen53 |
| 45 | 0.154±?** | Anthracite coal | Schmidt et al.54 |
| 46 | 0.152±0.025 | Wood | Beukens55 |
| 47 | 0.142±0.023 | Anthracite | Vogel et al.56 |
| 48 | 0.142±0.028 | CaC2 from coal | Gurfinkel57 |
| 49 | 0.14±0.02 | Marble | Schleicher et al.58 |
| 50 | 0.13±0.03 | Shell (Mytilus edulis) | Nadeau et al.59 |
| 51 | 0.130±0.009 | Graphite | Gurfinkel60 |
| 52 | 0.128±0.056 | Graphite | Vogel et al.61 |
| 53 | 0.125±0.060 | Calcite | Vogel et al.62 |
| 54 | 0.12±0.03 | Foraminifera (N. pachyderma) | Nadeau et al.63 |
| 55 | 0.112±0.057 | Bituminous coal | Kitagawa et al.64 |
| 56 | 0.1±0.01 | Graphite (NBS) | Donahue et al.65 |
| 57 | 0.1±0.05 | Petroleum, cracked | Gillespie and Hedges66 |
| 58 | 0.098±0.009* | Marble | Schleicher et al.67 |
| 59 | 0.092±0.006 | Wood | Kirner, Taylor, and Southon68 |
| 60 | 0.09–0.18* (range) | Graphite powder | Aerts-Bijma et al.69 |
| 61 | 0.09–0.13* (range) | Fossil CO2 gas | Aerts-Bijma et al.70 |
| 62 | 0.089±0.017 | Graphite | Arnold et al.71 |
| 63 | 0.081±0.019 | Anthracite | Beukens72 |
| 64 | 0.08±? | Natural graphite | Donahue et al.73 |
| 65 | 0.080±0.028 | Cararra marble | Nadeau et al.74 |
| 66 | 0.077±0.005 | Natural gas | Beukens75 |
| 67 | 0.076±0.009 | Marble | Beukens76 |
| 68 | 0.074±0.014 | Graphite powder | Kirner et al.77 |
| 69 | 0.07±? | Graphite | Kretschmer et al.78 |
| 70 | 0.068±0.028 | Calcite (Icelandic double spar) | Nadeau et al.79 |
| 71 | 0.068±0.009 | Graphite (fresh surface) | Schmidt et al.80 |
| 72 | 0.06–0.11 (range) | Graphite (200 Ma) | Nakai et al.81 |
| 73 | 0.056±? | Wood (selected data) | Kirner et al.82 |
| 74 | 0.05±0.01 | Carbon | Wild et al.83 |
| 75 | 0.05±? | Carbon-12 (mass sp.) | Schmidt et al.84 |
| 76 | 0.045–0.012 (m0.06) | Graphite | Grootes et al.85 |
| 77 | 0.04±?* | Graphite rod | Aerts-Bijma et al.86 |
| 78 | 0.04±0.01 | Graphite (Finland) | Bonani et al.87 |
| 79 | 0.04±0.02 | Graphite | Van der Borg88 |
| 80 | 0.04±0.02 | Graphite (Ceylon) | Bird et al.89 |
| 81 | 0.036±0.005 | Graphite (air) | Schmidt et al.90 |
| 82 | 0.033±0.013 | Graphite | Kirner et al.91 |
| 83 | 0.03±0.015 | Carbon powder | Schleicher et al.92 |
| 84 | 0.030±0.007 | Graphite (air redone) | Schmidt et al.93 |
| 85 | 0.029±0.006 | Graphite (argon redone) | Schmidt et al.94 |
| 86 | 0.029±0.010 | Graphite (fresh surface) | Schmidt et al.95 |
| 87 | 0.02±? | Carbon powder | Pearson et al.96 |
| 88 | 0.019±0.009 | Graphite | Nadeau et al.97 |
| 89 | 0.019±0.004 | Graphite (argon) | Schmidt et al.98 |
| 90 | 0.014±0.010 | CaC2 (technical grade) | Beukens99 |
*Estimated from graph
**Lowest value of multiple dates
This large spread in 14C content invites an explanation. A third observation, although weaker that the first two, is that the distribution of values for non-biogenic material displays a peak offset from zero. This may provide a hint that carbon never cycled through living organisms—in most cases locked away in Precambrian geological settings—may actually contain a low level of intrinsic 14C.
Coping with Paradigm Conflict
How do the various 14C laboratories around the world deal with the reality that they measure significant amounts of 14C, far above the detection threshold of their instruments, in samples that should be 14C dead according to the standard geological timescale? A good example can be found in a recent paper by Nadeau et al.100 entitled, “Carbonate 14C background: Does it have multiple personalities?” The authors are with the Leibnitz Laboratory at Christian-Albrechts University in Kiel, Germany. Many of the samples they analyze are shells and foraminifera tests from sediment cores. It would very useful to them if they could extend the range for which they could date such biological carbonate material from roughly 40,000 years ago (according to their uniformitarian assumptions), corresponding to about 1 pmc, toward the 0.002 pmc limit of their AMS instrument, corresponding to about 90,000 years in terms of uniformitarian assumptions. The reason they are presently stuck at this 40,000-year barrier is that they consistently and reproducibly measure 14C levels approaching 1 pmc in shells and foraminifera from depths in the record where, according to the standard geological timescale, there should be no detectable 14C.
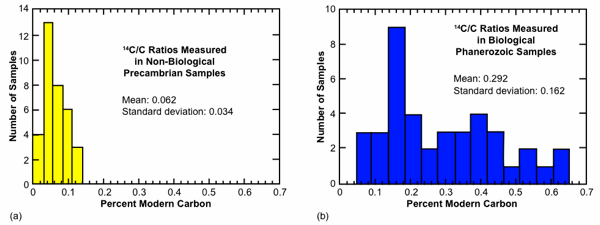
Fig. 2. Distribution of 14C values for (a) non-biogenic samples and (b) biogenic samples from Table 1. Given their position in the geological record, all these samples should contain no detectable 14C according to the standard geological time scale.
Their paper reports detailed studies they have carried out to attempt to understand the source of this 14C. They investigated shells from a late Pleistocene coring site in northwestern Germany dated by U/Th methods at 120,000 years. The mean 14C levels measured in the shells of six different species of mussels and snails varied from 0.1 to 0.5 pmc. In the case of one species, Spisula subtruncata, measurements were made on both the outside and inside of the shell of a single individual specimen. The average 14C value for the outside of the shell was 0.3 pmc, while for the inside it was 0.67. At face value, this suggests the 14C/C ratio more than doubled during the lifetime of this organism. Most of their foraminifera were from a Pleistocene core from the tropical Atlantic off the northwest coast of Africa dated at 455,000 years. The foraminifera from this core showed a range of 14C values from 0.16 to 0.4 pmc with an average, taken over 115 separate measurements, of 0.23 pmc. A benthic species of foraminifera from another core, chosen because of its thick shell and smooth surface in the hope its “contamination” would be lower, actually had a higher average 14C level of 0.58 pmc!
The authors then performed a number of experiments involving more aggressive pre-treatment of the samples to attempt to remove contamination. These included progressive stepwise acid hydrolization of the carbonate samples to CO2 gas and 14C measurement of each of four separate gas fractions. They found a detectable amount of surface contamination was present in the first fraction collected, but it was not large enough to make the result from the final gas fraction significantly different from the average value. They also leached samples in hydrochloric acid for two hours and cracked open the foraminifera shells to remove secondary carbonate from inside, but these procedures did not significantly alter the measured 14C values.
The authors summarize their findings in the abstract of their paper as follows,
The results … show a species-specific contamination that reproduces over several individual shells and foraminifera from several sediment cores. Different cleaning attempts have proven ineffective, and even stronger measures such as progressive hydrolization or leaching of the samples prior to routine preparation, did not give any indication of the source of contamination.
In their conclusion they state,
The apparent ages of biogenic samples seem species related and can be reproduced measuring different individuals for larger shells or even different sediment cores for foraminifera. Although tests showed some surface contamination, it was not possible to reach lower 14C levels through cleaning, indicating the contamination to be intrinsic to the sample.
They continue,
So far, no theory explaining the results has survived all the tests. No connection between surface structure and apparent ages could be established.
The measurements reported in this paper obviously represent serious anomalies relative to what should be expected in the uniformitarian framework. There is a clear conflict between the measured levels of 14C in these samples and the dates assigned to the geological setting by other radioisotope methods. The measured 14C levels, however, are far above instrument threshold and also appear to be far above contamination levels arising from sample processing. Moreover, the huge difference in 14C levels among species co-existing in the same physical sample violates the assumption that organisms living together in the same environment should share a common 14C/C ratio. The position the authors take in the face of these conflicts is that this 14C, which should not be present according to their framework, represents “contamination” for which they currently have no explanation. On the other hand, in terms of the framework of a young earth and a recent global Flood, these measurements provide important clues these organisms are much younger than the standard geological timescale would lead one to suspect.
This same approach of treating measurable and reproducible 14C values in samples that ought to be 14C dead, given their position in the geological record, as “contamination” is found throughout the current literature. Bird,101 for example, freely acknowledge “contamination” in old samples leads to a “radiocarbon barrier:”
Detecting sample contamination and verifying the reliability of the ages produced also becomes more difficult as the age of the sample increases. In practice this means that many laboratories will only quote 14C ages to about 40 ka BP (thousands of 14C years before present), with ages greater than this generally considered to be “infinite,” or indistinguishable from procedural blanks. The so-called “radiocarbon barrier” and the difficulty of ensuring that ages are reliable at <1% modern carbon levels has limited research in many disciplines.
This statement is in the context of a high precision AMS facility the authors use, capable of measuring 14C levels in the range of <<0.01 pmc.
In their paper they describe a strategy for eliminating various types of genuine contamination commonly associated with charcoal samples. A main component of this strategy is a stepped combustion procedure in which the sample is oxidized to CO2 in a stepwise manner, at temperatures of 330 °C, 630 °C, and 850 °C, with the resulting CO2 fractions analyzed separately using AMS. Oxidation of most of any surficial contamination generally occurs at the lowest temperature, and the 14C level of the highest temperature fraction is generally considered the one representing the least contaminated portion of the sample. The variation among the three fractions is considered a general indicator of the overall degree of contamination. They apply this approach to analysis of charcoal from one of the early sites of human occupation in Australia.
Included in their paper is considerable discussion of what is known as a “procedural blank,” or a sample that represents effectively infinite 14C age. For this they use what they refer to as “radiocarbon-dead” graphite from Ceylon. They apply their stepped combustion procedure, using only the highest temperature fraction, on 14 such graphite samples to get a composite value of 0.04±0.02 pmc for this background material. They note that a special pre-treatment they use for charcoal samples applied to four of the 14 samples yielded results indistinguishable from the other 10 graphite samples that had no pre-treatment. They further note that sample size variation between 0.1 and 2.2 mg among the 14 samples also made no difference in the results. From this they acknowledge, “the few 14C atoms observed may already be present in the Ceylon graphite itself.” Indeed, they offer no explanation for the fact that this graphite displays 14C levels well above the detection threshold of their AMS system other than it might be inherent to the graphite itself.
Measuring notable levels of 14C in samples intended as procedural blanks or “background” samples is a phenomenon that has persisted from the earliest days of AMS down to the present time. For example, Vogel, Nelson, and Southon102 describe their thorough investigation of the potential sources and their various contributions to the 14C background in their AMS system. The material they used for the blank in their study was anthracite coal from a deep mine in Pennsylvania. An important part of their investigation was variation of the sample size of the blank by a factor of 2000, from 10 mg to 20 mg. They found that samples 500 mg and larger displayed a 14C concentration of 0.44±0.13 pmc, independent of sample size, implying this 14C was intrinsic to the anthracite material itself. For samples smaller than 500 mg, the measured 14C could be explained in terms of this intrinsic 14C, plus contamination by a constant amount of modern carbon that seemed to be present regardless of sample size. After many careful experiments, the authors concluded that the main source of this latter contamination was atmospheric CO2 adsorbed within the porous Vicor glass used to encapsulate the coal sample in its combustion to CO2 at 900 °C. Another source of smaller magnitude was CO2 and CO adsorbed on the walls of the graphitization apparatus retained from reduction of earlier samples. It was found that filling the apparatus with water vapor at low pressure and then evacuating the apparatus before the next graphitization mostly eliminated this memory effect. Relative to these two sources, measurements showed that storage and handling of the samples, contamination of the copper oxide used in combustion, and contamination of the iron oxide powder used in the graphitization were effectively negligible. And when the sample size was greater than 500 mg, the intrinsic 14C in the coal swamped all the sources of real 14C contamination. Rather than deal with the issue of the nature of the 14C intrinsic to the anthracite itself, the authors merely refer to it as “contamination of the sample in situ”, “not [to be] discussed further.”
As it became widely appreciated that many high carbon samples, which ought to be 14C “dead” given their position in the geological record, had in fact 14C levels far above AMS machine thresholds, the approach was simply to search for specific materials that had as low a 14C background level as possible.For example, Beukens,103 at the IsoTrace Laboratory at the University of Toronto, describes measurements on two samples that, from his experience at that time, displayed exceptionally low background 14C levels. He reports 0.077±0.005 pmc from a sample of industrial CO2 obtained by combustion of natural gas and 0.076±0.009 pmc from Italian Carrara marble. Previously for his blank material he had used an optical grade calcite (Iceland spar) for which he measured a 14C level of 0.15 to 0.13 pmc. He emphasizes that the pre-treatment, combustion, and hydrolysis techniques applied to these new samples were identical to those normally applied to samples submitted for analysis to his laboratory and these techniques had not changed appreciably in the previous five years. He states,
The lower 14C levels in these [more recent] measurements should therefore be attributed entirely to the lower intrinsic 14C contamination of these samples and not to changes in sample preparation or analysis techniques.
Note that he indeed considers the 14C in all these materials to be “intrinsic,” but he has to call it “contamination.” In his search for even better procedural blanks, he tested two standard blank materials, a calcite and an anthracite coal, used by the Geological Survey of Canada in their beta decay counting 14C laboratory. These yielded 14C levels of 0.54±0.04 pmc for the calcite and 0.36±0.03 pmc for the coal. Beukens noted with moderate alarm that the background corrections being made by many decay-counting radiocarbon dating facilities that had not checked the intrinsic 14C content of their procedural blanks by AMS methods were probably quoting ages systematically older than the actual ages. His AMS analysis of the samples from the Geological Survey of Canada “clearly shows these samples are not 14C-free” since these levels were markedly higher than those from his own natural gas and marble blanks.
AMS analyses reveal carbon from fossil remains of living organisms, regardless of their position in the geological record, consistently contains 14C levels far in excess of the AMS machine threshold, even when extreme pre-treatment methods are applied. Experiments in which the sample size is varied argue compellingly that the 14C is intrinsic to the fossil material and not a result of handling or pre-treatment. These conclusions continue to be confirmed in the very latest peer-reviewed papers. Moreover, even non-organic carbon samples appear consistently to yield 14C levels well above machine threshold. Graphite samples formed under metamorphic and reducing conditions in Precambrian limestone environments commonly display 14C values on the order of 0.05 pmc. Most AMS laboratories are now using such Precambrian graphite for their procedural blanks. A good question is what possibly could be the source of the 14C in this material? We conclude that the possibility this 14C is primordial is a reasonable one. Finding 14C in diamond formed in the earth’s mantle would provide support for such a conclusion. Establishing that non-organic carbon from the mantle and from Precambrian crustal settings consistently contains inherent 14C well above the AMS detection threshold would, of course, argue the earth itself is less than 100,000 years old, which is orders of magnitude younger than the 4.56 Ga currently believed by the uniformitarian community.
Results of RATE 14C AMS Analyses
Table 2 summarizes the results from ten coal samples prepared by our RATE team and analyzed by one of the foremost AMS laboratories in the world. These measurements were performed using the laboratory’s “high precision” procedures which involved four runs on each sample, the results of which were combined as a weighted average and then reduced by 0.077±0.005 pmc to account for a “standard background” of contamination believed to be introduced by sample processing. This standard background value is obtained by measuring the 14C in a purified natural gas. Subtraction of this background value is justified by the assumption that it must represent contamination. Fig. 3 displays these AMS analysis results in histogram format.
Table 2. Results of AMS 14C analysis of ten RATE coal samples.
| Sample | Coal Seam Name | State | County | Geological Interval | 14C/C (pmc) |
|---|---|---|---|---|---|
| DECS-1 | Bottom | Texas | Freestone | Eocene | 0.30±0.03 |
| DECS-11 | Beulah | North Dakota | Mercer | Eocene | 0.20±0.02 |
| DECS-25 | Pust | Montana | Richland | Eocene | 0.27±0.02 |
| DECS-15 | Lower Sunnyside | Utah | Carbon | Cretaceous | 0.35±0.03 |
| DECS-16 | Blind Canyon | Utah | Emery | Cretaceous | 0.10±0.03 |
| DECS-28 | Green | Arizona | Navajo | Cretaceous | 0.18±0.02 |
| DECS-18 | Kentucky #9 | Kentucky | Union | Pennsylvanian | 0.46±0.03 |
| DECS-21 | Lykens Valley #2 | Pennsylvania | Columbia | Pennsylvanian | 0.13±0.02 |
| DECS-23 | Pittsburgh | Pennsylvania | Washington | Pennsylvanian | 0.19±0.02 |
| DECS-24 | Illinois #6 | Illinois | Macoupin | Pennsylvanian | 0.29±0.03 |
Details of RATE Sample Selection and Analysis
The ten samples in Table 2 were obtained from the U. S. Department of Energy Coal Sample Bank maintained at Penn State University. The coals in this bank are intended to be representative of the economically important coalfields of the United States. The original samples were collected in 400-pound quantities from recently exposed areas of active mines, where they were placed in 30-gallon steel drums with high-density gaskets and purged with argon. As soon as feasible after collection, these large samples were processed to obtain representative 300 g samples with 0.85 mm particle size (20 mesh). These smaller 300 g samples were sealed under argon in foil multilaminate bags and have since been kept in refrigerated storage at 3 °C. We selected ten of the 33 coals available with an effort to obtain good representation geographically as well as with respect to depth in the geological record. Our ten samples include three Eocene, three Cretaceous, and four Pennsylvanian coals.
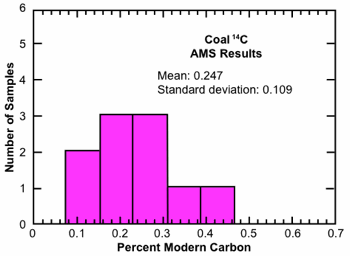
Fig. 3. Histogram representation of AMS 14C analysis of ten coal samples undertaken by RATE 14C research project.
The 14C analysis at the AMS laboratory we selected involves first processing the coal samples to make graphite targets and then counting the relative numbers of atoms from the different carbon isotopes in the accelerator mass spectrometer system. The accelerator generates an intense ion beam that ionizes the graphite on the target, while the mass spectrometer uses electric and magnetic fields to separate different atomic species by mass and charge and counts the numbers of triply ionized 14C, 13C, and 12C atoms. The sample processing consists of three steps:combustion, acetylene synthesis, and graphitization.The coal samples are first combusted to CO2 and then converted to acetylene using a lithium carbide synthesis process. The acetylene is then dissociated in a high voltage AC electrical discharge to produce a circular disk of graphite on spherical aluminum pellets that represent the targets for the AMS system. Four separate targets are produced for each sample. Every target is analyzed in a separate AMS run with two modern carbon standards (NBS I oxalic acid). Each target is then analyzed on 16 different spots (organized on two concentric circles). The advantage of this procedure over a single high precision measurement is that a variance check (typically a T-test) can be performed for the 16 spots on each target. If an individual target fails this variance test, it is rejected. While this has advantages for any kind of sample, it is particularly useful for samples with very low 14C levels because they are especially sensitive to contamination. While great care is taken to prevent target contamination after the graphitization step, it nevertheless can happen. Any contaminated spot or any contaminated target would bias the average. This variance test attempts to identify and eliminate this source of error.
Table 3 gives the measurements in pmc from the four separate targets for our ten coal samples. The numbers in parentheses are the percent errors, calculated from the 14C count rate of the sample and the two NBS standards and from the transmission of errors in the 12C and 13C current measurements of the sample and two standards. The composite results in Table 2 represent the weighted averages of these numbers in Table 3 and the subtraction of a standard background of 0.077±0.005 pmc.
Table 3. Detailed AMS 14C measurements for 10 RATE coal samples in pmc.
| Sample | Target 1 | Target 2 | Target 3 | Target 4 |
|---|---|---|---|---|
| DECS-1 | 0.398 (12.0%) | 0.355 (13.2%) | 0.346 (15.1%) | 0.346 (15.1%) |
| DECS-11 | 0.237 (18.2%) | 0.303 (14.8%) | 0.292 (17.8%) | 0.294 (17.2%) |
| DECS-25 | 0.342 (13.3%) | 0.359 (15.3%) | 0.352 (14.2%) | 0.328 (14.8%) |
| DECS-15 | 0.416 (13.1%) | 0.465 (12.2%) | 0.467 (12.2%) | 0.377 (13.6%) |
| DECS-16 | 0.184 (25.0%) | 0.233 (21.8%) | 0.141 (38.4%) | 0.163 (34.0%) |
| DECS-28 | 0.203 (18.3%) | 0.379 (14.5%) | 0.204 (21.2%) | 0.204 (21.2%) |
| DECS-18 | 0.533 (11.8%) | 0.539 (11.4%) | 0.492 (11.6%) | 0.589 (10.0%) |
| DECS-21 | 0.183 (22.0%) | 0.194 (20.0%) | 0.230 (18.2%) | 0.250 (18.0%) |
| DECS-23 | 0.225 (18.1%) | 0.266 (13.8%) | 0.246 (18.7%) | 0.349 (13.2%) |
| DECS-24 | 0.334 (19.7%) | 0.462 (17.5%) | 0.444 (13.4%) | 0.252 (25.8%) |
The background standard of this AMS laboratory is CO2 from purified natural gas that provides their background level of 0.077±0.005 pmc. This same laboratory obtains values of 0.076±0.009 pmc and 0.071±0.009 pmc, respectively, for Carrara Marble (IAEA Standard Radiocarbon Reference Material C1) and optical-grade calcite from Island spar. They claim this is one of the lowest background levels quoted among AMS labs, and they attribute this low background to their special graphitization technique. They emphasize backgrounds this low cannot be realized with any statistical significance through only one or two measurements, but many measurements are required to obtain a robust determination.
The laboratory has carefully studied the sources of error within its AMS hardware, and regular tests are performed to ensure these remain small. According to these studies, errors in the spectrometer are very low and usually below the detection limit since the spectrometer is energy dispersive and identifies the ion species by energy loss. The detector electronic noise, the mass spectrometric inferences (the E/q and mE/q2 ambiguities), and the cross contamination all contribute less than 0.0004 pmc to the background. Ion source contamination as a result of previous samples (ion source memory) is a finite contribution because 50–80% of all sputtered carbon atoms are not extracted as carbon ions and are therefore dumped into the ion source region. To limit this ion source memory effect, the ion source is cleaned every two weeks and critical parts are thrown away. This keeps the ion source contamination at approximately 0.0025 pmc for the duration of a two-week run. Regular spot checks of these contributions are performed with a zone-refined, reactor-grade graphite sample (measuring 14C/12C ratios), and blank aluminum target pellets (measuring 14C only).
The laboratory claims most of their quoted system background arises from sample processing. This processing involves combustion (or hydrolysis in the case of carbonate samples), acetylene synthesis, and graphitization. Yet careful and repeated analysis of their methods over more than 15 years have convinced them that very little contamination is associated with the combustion or hydrolysis procedures and almost none with their electrical dissociation graphitization process. By elimination they conclude that the acetylene synthesis must contribute almost all of the system background. But they can provide little tangible evidence it actually does. Our assessment from the information we have is that the system background arises primarily from 14C intrinsic to the background standards themselves. The values we report in Table 2 and Fig. 3 nevertheless include the subtraction of the laboratory’s standard background. In any case, the measured 14C/C values are notably above their background value.
Making Sense of the 14C Data
How does one make sense of these 14C measurements that yield a uniformitarian ages of 40,000–60,000 years for organic samples, such as our coal samples, that have uniformitarian ages of 40–350 million years based on long half-life isotope methods applied to surrounding host rocks? Clearly there is an inconsistency. Our hypothesis is that the source of the discrepancy is the interpretational framework that underlies these methods. Could the proposition, articulated 180 years ago by Charles Lyell, that “the present is the key to the past” be suspect? Could the standard practice employed all these years by earth scientists and others of extrapolating the processes and rates observed in today’s world into the indefinite past not be reliable after all? As authors of this paper we are convinced that there is abundant observational evidence in the geological record that the earth has experienced a global tectonic catastrophe of immense magnitude that is responsible for most of the Phanerozoic geological record. We are persuaded it is impossible any longer to claim that geological processes and rates observable today can account for the majority of the Phanerozoic sedimentary record. To us the evidence is overwhelming that global scale processes operating at rates much higher than any observable on earth today are responsible for this geological change.104,105,106,107 Not only are the 14C data at odds with the standard geological timescale, but the general character of the sedimentary and tectonic record is as well. We realize for many such a view of the geological data is new, or at least controversial. For those new to this possibility we urge reading of some of our papers on this topic (for example, Austin108, Baumgardner109,110,111). We are convinced that not only do the observations strongly support this interpretation of the geological record, but the theoretical framework also now exists to explain it (Baumgardner112,113,114). Our approach for making sense of these 14C data, therefore, is to do so in the light of a major discontinuity in earth history in its not so distant past, an event we correlate with the Flood described in the Bible as well as in many other ancient documents.
What was the Pre-Flood 14C Level?
What sorts of 14C/C ratios might we expect to find today in organic remains of plants and animals buried in a single global cataclysm correlated with all but the latter part of the Phanerozoic geological record (that is, Cambrian to middle-upper Cenozoic)? Such a cataclysm would have buried a huge amount of carbon from living organisms to form today’s coal, oil, and oil shale, probably most of the natural gas, and some fraction of today’s fossiliferous limestone. Estimates for the amount of carbon in this inventory are at least a factor of 100 greater than what currently resides in the biosphere.115,116,117 This implies the biosphere just prior to the cataclysm would have had at least 100 times the total carbon relative to our world today. Living plants and animals would have contained most of this biospheric carbon, with only a tiny fraction of the total in the atmosphere. The vast majority of this carbon would have been 12C, since even today only about one carbon atom in a trillion is 14C.
To estimate the pre-cataclysm 14C/C ratio we of course require an estimate for the amount of 14C. As a starting point we might assume the total amount was similar to what exists in today’s world. If that were the case, and this 14C were distributed uniformly, the resulting 14C/C ratio would be about 1/100 of today’s level, or about 1 pmc. This follows from the fact that 100 times more carbon in the biosphere would dilute the available 14C and cause the biospheric 14C/C ratio to be 100 times smaller than today. But this value of 1 pmc is probably an upper limit because there are reasons to suspect the total amount of 14C just prior to the cataclysm was less, possibly much less, than exists today. Two important issues come into play here in regard to the amount of pre-Flood 14C—namely, the initial amount of 14C after creation and the 14C production rate in the span of time between creation and the Flood catastrophe. We have seen already there are hints of primordial 14C in non-biogenic Precambrian materials at levels on the order of 0.05 pmc. This provides a clue that the 14C/C ratio in everything containing carbon just after creation might have been on the order of 0.1 pmc. But it is also likely 14C was added to the biosphere between creation and the Flood. The origin of 14C in today’s world is by cosmic ray particles in the upper atmosphere changing a proton in the nucleus of a 14N atom into a neutron to yield a 14C atom. Just what the 14C production rate prior to the cataclysm might have been is not easily constrained. It could well have been lower than today if the earth’s magnetic field strength were higher and resulting cosmic ray flux lower. But perhaps it was not. In any case, given the 5,730-year half-life of 14C, it is almost certain the less than 2,000 year interval between creation and the Flood was insufficient for 14C to have reached an equilibrium level in the biosphere. If the 14C production rate itself was roughly constant, then the 14C/C ratio in the atmosphere would have been a steadily increasing function of time across this interval. Hence, we conclude the pre-Flood 14C/C ratios were likely no greater than 1 pmc but also highly variable, especially in the case of plants, depending on when during the interval they generated their biomass.
In addition to the preceding considerations, we must also account for the 14C decay that has occurred since the cataclysm. Assuming a constant 14C half-life of 5,730 years, the 14C/C ratio in organic material buried, say, 5,000 years ago would be reduced by an additional factor of 0.55. When we combine all these factors, we conclude it is not at all surprising organic materials buried in the cataclysm should display the roughly 0.05–0.5 pmc we actually observe. We note that when these considerations are included, especially the larger pre-cataclysm carbon inventory, a 14C/C value of 0.24 pmc, for example, is consistent with an actual age of 5,000 years. By contrast, when these considerations are not taken into account, the uniformitarian formula, pmc = 100 × 2–t/5730, displayed in graphical form in Figure 1, yields an age of 50,000 years. Yet in either case, the 14C ages are still typically orders of magnitude less than those provided by the long half-life radioisotope methods.
In this context it is useful to note that 14C/C levels must have increased dramatically and rapidly just after the cataclysm, assuming near modern rates of 14C production in the upper atmosphere, due to the roughly hundredfold reduction in the amount of carbon in the biospheric inventory. The large variation in 14C levels between species as well as from the outside to the inside of a single shell as reported by Nadeau et al.118 indeed seems to suggest significant spatial and temporal variations in this dynamic period during which the planet was recovering from the cataclysm.
Effect of Accelerated Decay on Pre-Flood 14C
Other RATE projects are building a compelling case that episodes of accelerated nuclear decay must have accompanied the creation of the earth as well as the Genesis Flood.119,120,121 We believe several billions of years worth of cumulative decay at today’s rates must have occurred for isotopes such as 238U during the creation of the physical earth, and we now suspect a significant amount of such decay likely also occurred during the Flood cataclysm. An important issue then arises as to how an episode of accelerated decay during the Flood might have affected a short half-life isotope like 14C. The fact that significant amounts of 14C are measured routinely in fossil material from organisms alive before the cataclysm argues persuasively that only a modest amount of accelerated 14C decay occurred during the cataclysm itself. This suggests the possibility that the fraction of unstable atoms that decayed during the acceleration episode for all of the unstable isotopes might have been roughly the same. If the fraction were exactly the same, this would mean that the acceleration in years for each isotope was proportional to the isotope’s half-life. In this case, if 40K, for example, underwent 400 Ma of decay during the Flood relative to a present half-life of 1250 Ma, then 14C would have undergone (400/1250)*5730 years = 1,834 years of decay during the Flood. This amount of decay represents 1–2–(1834/5730) = 20% reduction in 14C as a result of accelerated decay. This is well within the uncertainty of the level of 14C in the pre-Flood world so it has little impact on the larger issues discussed in this paper.
Discussion
The initial vision that high precision AMS methods should make it possible to extend 14C dating of organic materials back as far as 90,000 years has not been realized. The reason seems to be clear. Few, if any, organic samples can be found containing so little 14C! This includes samples uniformitarians presume to be millions, even hundreds of millions, of years old. At face value, this ought to indicate immediately, entirely apart from any consideration of a Flood catastrophe, that life has existed on earth for less than 90,000 years. Although repeated analyses over the years have continued to confirm the 14C is an intrinsic component of the sample material being tested, such 14C is still referred to as “contamination” if it is derived from any part of the geological record deemed older than about 100,000 years. To admit otherwise would fatally undermine the uniformitarian framework. For the creationist, however, this body of data represents obvious support for the recent creation of life on earth. Significantly, the research and data underpinning the conclusion that 14C exists in fossil material from all portions of the Phanerozoic record are already established in the standard peer-reviewed literature. And the work has been performed largely by uniformitarians who hold no bias whatever in favor of this outcome. The evidence is now so compelling that additional AMS determinations by creationists on samples from deep within the Phanerozoic record can only make the case marginally stronger than it already is.
Indeed, the AMS results for our ten coal samples, as summarized in Table 2 and Fig. 3, fall nicely within the range for similar analyses reported in the radiocarbon literature, as presented in Table 1 and Fig. 2(b). Not only are the mean values of the two data sets almost the same, but the variances are also similar. Moreover, when we average the results from our coal samples over geological interval, we obtain mean values of 0.26 pmc for Eocene, 0.21 for Cretaceous, and 0.27 for Pennsylvanian that are remarkably similar to one another. These results, limited as they are, indicate little difference in 14C level as a function of position in the geological record. This is consistent with the young-earth view that the entire fossil record up to somewhere within the middle-upper Cenozoic is the product of a single recent global catastrophe. On the other hand, an explanation for the notable variation in 14C level among the ten samples is not obvious. One possibility is that the 14C production rate between creation and the Flood was sufficiently high that the 14C levels in the pre-Flood biosphere increased from, say, 0.1 pmc at creation to perhaps as much as 1 pmc just prior to the Flood. Plant material that grew early during this period and survived until the Flood would then contain low levels of 14C, while plant material produced by photosynthetic processes just prior to the cataclysm would contain much higher values. This situation would prevail across all ecological zones on the planet, and so the large variations in 14C levels would appear within all stratigraphic zones that were a product of the Flood.
Moreover, in contrast to the uniformitarian outlook that 14C in samples older than late Pleistocene must be contamination and therefore is of little or no scientific interest, such 14C for the creationist potentially contains vitally important clues to the character of the pre-Flood world. The potential scientific value of these 14C data in our opinion merits a serious creationist research effort to measure the 14C content in fossil organic material from a wide variety of pre-Flood environments, both marine and terrestrial. Systematic variations in 14C levels, should they be discovered, conceivably could provide important constraints on the time history of 14C levels and 14C production, the pattern of atmospheric circulation, the pattern of oceanic circulation, and the carbon cycle in general in the pre-Flood world.
Furthermore, a careful study of the 14C content of carbon that has not been cycled through living organisms, especially carbonates, graphites, and diamonds from environments believed to pre-date life on earth, could potentially place very strong constraints on the age of the earth itself. The data already present in the peer-reviewed radiocarbon literature suggests there is indeed intrinsic 14C in such materials that cannot be attributed to contamination. If this conclusion proves robust, these reported 14C levels then place a hard limit on the age of the earth of less than 100,000 years, even when viewed from a uniformitarian perspective. We believe a creationist research initiative focused on this issue deserves urgent support.
Conclusion
The careful investigations performed by scores of researchers in more than a dozen AMS facilities in several countries over the past 20 years to attempt to identify and eliminate sources of contamination in AMS 14C analyses have, as a by-product, served to establish beyond any reasonable doubt the existence of intrinsic 14C in remains of living organisms from all portions of the Phanerozoic record. Such samples, with “ages” from 1–500 Ma as determined by other radioisotope methods applied to their geological context, consistently display 14C levels that are far above the AMS machine threshold, reliably reproducible, and typically in the range of 0.1–0.5 pmc. But such levels of intrinsic 14C represent a momentous difficulty for uniformitarianism. A mere 250,000 years corresponds to 43.6 half-lives for 14C. One gram of modern carbon contains about 6 × 1010 14C atoms, and 43.6 half-lives worth of decay reduces that number by a factor of 7 × 10–14. Not a single atom of 14C should remain in a carbon sample of this size after 250,000 years (not to mention one million or 50 million or 250 million years). A glaring (thousand-fold) inconsistency that can no longer be ignored in the scientific world exists between the AMS-determined 14C levels and the corresponding rock ages provided by 238U, 87Rb, and 40K techniques. We believe the chief source for this inconsistency to be the uniformitarian assumption of time-invariant decay rates. Other research reported by our RATE group also supports this conclusion.122,123,124 Regardless of the source of the inconsistency, the fact that 14C, with a half-life of only 5,730 years, is readily detected throughout the Phanerozoic part of the geological record argues the half billion years of time uniformitarians assign to this portion of earth history is likely incorrect. The relatively narrow range of 14C/C ratios further suggests the Phanerozoic organisms may all have been contemporaries and that they perished simultaneously in the not so distant past. Finally, we note there are hints that 14C currently exists in carbon from environments sealed from biospheric interchange since very early in the earth history. We therefore conclude the 14C evidence provides significant support for a model of earth’s past involving a recent global Flood cataclysm and possibly also for a young age for the earth itself.
Acknowledgments
We would like to thank Paul Giem for the helpful input he provided in this project. We would also like to express earnest appreciation to the RATE donors who provided the financial means to enable us to undertake 14C analysis of our own suite of samples.
Bibliography
Beukens, R. P., D. M. Gurfinkel, and H. W. Lee. 1992. Progress at the Isotrace Radiocarbon Facility. Radiocarbon 28:229–236.
Donahue, D. J., A. J. T. Jull, and L. J. Toolin. 1990. Radiocarbon measurements at the University of Arizona AMS Facility. Nuclear Instruments and Methods in Physics Research B 52:224–228.
Donahue, D. J., A. J. T. Jull, and T. H. Zabel. 1984. Results of radioisotope measurements at the NSF-University of Arizona tandem Accelerator Mass Spectrometer facility. Nuclear Instruments and Methods in Physics Research B 5:162–166.
Footnotes
- Giem, P. 2001. Carbon-14 content of fossil carbon. Origins 51:6–30.
- Whitelaw, R. L. 1970. Time, life, and history in the light of 15,000 radiocarbon dates. Creation Research Society Quarterly 7 no. 1:56–71.
- Snelling, A. A. 1997. Radioactive “dating” in conflict! Fossil wood in ancient lava flow yields radiocarbon. Creation Ex Nihilo 20 no. 1:24–27.
- Snelling, A. A. 1998. Stumping old-age dogma: Radiocarbon in an “ancient” fossil tree stump casts doubt on traditional rock/fossil dating. Creation Ex Nihilo 20 no. 4:48–51.
- Snelling, A. A. 1999. Dating dilemma: Fossil wood in ancient sandstone. Creation Ex Nihilo 21 no. 3:39–41.
- Snelling, A. A. 2000. Geological conflict: Young radiocarbon date for ancient fossil wood challenges fossil dating. Creation Ex Nihilo 22 no. 2:44–47.
- Snelling, A. A., 2000. Conflicting “ages” of Tertiary basalt and contained fossilized wood, Crinum, central Queensland, Australia. Creation Ex Nihilo Technical Journal 14 no. 2:99–122.
- Giem, Ref. 1.
- Giem, Ref. 1.
- Aerts-Bijma, A. T., H. A. J. Meijer, and J. van der Plicht. 1997. AMS sample handling in Groningen. Nuclear Instruments and Methods in Physics Research B 123:221–225.
- Beukens, R. P. 1990. High-precision intercomparison at Isotrace. Radiocarbon 32:335–339.
- Arnold, M., E. Bard, P. Maurice, and J. C. Duplessy. 1987. 14C dating with the Gif-sur-Yvette Tandetron Accelerator: Status report. Nuclear Instruments and Methods in Physics Research B 29:120–123.
- Schmidt, F. H., D. R. Balsley, and D. D. Leach. 1987. Early expectations of AMS: Greater ages and tiny fractions. One failure?—One success. Nuclear Instruments and Methods in Physics Research B 29:97–99.
- Nadeau, M. -J., P. M. Grootes, A. Voelker, F. Bruhn, A. Duhr, and A. Oriwall. 2001. Carbonate 14C background: Does it have multiple personalities? Radiocarbon 43 no. 2A:169–176.
- Beukens, Ref. 11.
- Nadeau et al., Ref. 14.
- Jull, A. J. T., D. J. Donahue, A. L. Hatheway, T. W. Linick, and L. J. Toolin. 1986. Production of graphite targets by deposition from CO/H2 for precision accelerator 14C measurements. Radiocarbon 28:191–197.
- Gulliksen, S. and M. S. Thomsen. 1992. Estimation of background contamination levels for gas counting and AMS target preparation in Trondheim. Radiocarbon 34:312–317.
- Gillespie, R. and R. E. M. Hedges. 1984. Laboratory contamination in radiocarbon Accelerator Mass Spectrometry. Nuclear Instruments and Methods in Physics Research B 5:294–296.
- Beukens, Ref. 11.
- Vogel, J. S., D. E. Nelson, and J. R. Southon. 1987. 14C background levels in an Accelerator Mass Spectrometry system. Radiocarbon 29:323–333.
- Vogel, Nelson, and Southon, Ref. 21.
- Grootes, P. M., M. Stuiver, G. W. Farwell, D. D. Leach, and F. H. Schmidt. 1986. Radiocarbon dating with the University of Washington Accelerator Mass Spectrometry system. Radiocarbon 28:237–245.
- Schleicher, M., P. M. Grootes, M. -J. Nadeau, and A. Schoon. 1998. The carbonate 14C background and its components at the Leibniz AMS facility. Radiocarbon 40:85–93.
- Nadeau et al., Ref. 14.
- Snelling, Ref. 3.
- Beukens, R. P. 1992. Radiocarbon Accelerator Mass Spectrometry: Background, precision, and accuracy. In Taylor, R. E., A. Long, and R. S. Kra (eds.). Radiocarbon after four decades: An interdisciplinary perspective, pp. 230–239. New York: Springer-Verlag.
- Nadeau et al., Ref. 14.
- Beukens, Ref. 27.
- Arnold et al., Ref. 12.
- Gulliksen and Thomsen, Ref. 18.
- Terrasi, F., L. Campajola, A. Brondi, M. Cipriano, A. D’Onofrio, E. Fioretto, M. Romano, C. Azzi, F. Bella, and C. Tuniz. 1990. AMS at the TTT-3 Tandem Accelerator in Naples. Nuclear Instruments and Methods in Physics Research B 52:259–262.
- Schleicher et al., Ref. 24.
- Schmidt et al., Ref. 13.
- McNichol, A. P., A. R. Gagnon, E. A. Osborne, D. L. Hutton, K. F. Von Reden, and R. J. Schneider. 1995. Improvements in procedural blanks at NOSAMS: Reflections of improvements in sample preparation and accelerator operation. Radiocarbon 37:683–691.
- Nadeau et al., Ref. 14.
- Beukens, Ref. 11.
- Schmidt et al., Ref. 13.
- Grootes et al., Ref. 23.
- Aertes-Bijma et al., Ref. 10.
- Nadeau et al., Ref. 14.
- Nadeau et al., Ref. 14.
- Donahue, D. J., J. W. Beck, D. Biddulph, G. S. Burr, C. Courtney, P. E. Damon, A. L. Hatheway, L. Hewitt, A. J. T. Jull, T. Lange, N. Lifton, R. Maddock, L. R. McHargue, J. M. O’Malley, and L. J. Toolin. 1997. Status of the NSF-Arizona AMS Laboratory. Nuclear Instruments and Methods in Physics Research B 123:51–56.
- McNichol et al., Ref. 35.
- Van der Borg, K., C. Alderliesten, A. F. M. de Jong, A. van den Brink, A. P. de Haas, H. J. H. Kersemaekers, and J. E. M. J. Raaymakers. 1997. Precision and mass fractionation in 14C analysis with AMS. Nuclear Instruments and Methods in Physics Research B 123:97–101.
- Gulliksen and Thomsen, Ref. 18.
- Gulliksen and Thomsen, Ref. 18.
- Nelson, D. E., J. S. Vogel, J. R. Southon, and T. A. Brown. 1986. Accelerator radiocarbon dating at SFU. Radiocarbon 28:215–222.
- Van der Borg et al., Ref. 45.
- Gulliksen and Thomsen, Ref. 18.
- Schleicher et al., Ref. 24.
- Kirner, D. L., R. Burky, R. E. Taylor, and J. R. Southon. 1997. Radiocarbon dating organic residues at the microgram level. Nuclear Instruments and Methods in Physics Research B 123:214–217.
- Gulliksen and Thomsen, Ref. 18.
- Schmidt et al., Ref. 13.
- Beukens, Ref. 11.
- Vogel et al., Ref.21.
- Gurfinkel, D. M. 1987. An assessment of laboratory contamination at the Isotrace radiocarbon facility. Radiocarbon 29:335–346.
- Schleicher et al., Ref. 24.
- Nadeau et al., Ref. 14.
- Gurfinkel, Ref. 57.
- Vogel et al., Ref. 21.
- Vogel et al., Ref. 21.
- Nadeau et al., Ref. 14.
- Kitagawa, H., T. Masuzawa, T. Makamura, and E. Matsumoto. 1993. A batch preparation method for graphite targets with low background for AMS 14C measurements. Radiocarbon 35:295–300.
- Donahue et al., Ref. 43.
- Gillespie and Hedges, Ref. 19.
- Schleicher et al., Ref. 24.
- Kirner, D. L., R. E. Taylor, and J. R. Southon. 1995. Reduction in backgrounds of microsamples for AMS 14C dating. Radiocarbon 37:697–704.
- Aerts-Bijma et al., Ref. 10.
- Aerts-Bijma et al., Ref. 10.
- Arnold et al., Ref. 12.
- Beukens, Ref. 27.
- Donahue et al., Ref. 43.
- Nadeau et al., Ref. 14.
- Beukens, Ref. 27.
- Beukens, Ref. 27.
- Kirner, Taylor, and Southon, Ref. 68.
- Kretschmer, W., G. Anton, M. Benz, S. Blasche, E. Erler, E. Finckh, L. Fischer, H. Kerscher, A. Kotva, M. Klein, M. Leigart, and G. Morgenroth. 1998. The Erlangen AMS facility and its applications in 14C sediment and bone dating. Radiocarbon 40:231–238.
- Nadeau et al., Ref. 14.
- Schmidt et al., Ref. 13.
- Nakai, N., T. Nakamura, M. Kimura, T. Sakase, S. Sato, and A. Sakai. 1984. Accelerator mass spectroscopy of 14C at Nagoya University. Nuclear Instruments and Methods in Physics Research B 5:171–174.
- Kirner et al., Ref. 52.
- Wild, E., R. Golser, P. Hille, W. Kutschera, A. Priller, S. Puchegger, W. Rom, and P. Steier. 1998. First 14C results for archaeological and forensic studies at the Vienna Environmental Research Accelerator. Radiocarbon 40:273–281.
- Schmidt et al., Ref. 13.
- Grootes et al., Ref. 23.
- Aerts-Bijma et al., Ref. 10.
- Bonani, G., H. -J. Hofmann, E. Morenzoni, M. Nessi, M. Suter, and W. Wölffi, 1986. The ETH/SIN dating facility: A status report. Radiocarbon 28:246–255.
- Van der Borg, Ref. 45.
- Bird, M. I., L. K. Ayliffe, L. K. Fifield, C. S. M. Turney, R. G. Cresswell, T. T. Barrows, and B. David, 1999. Radiocarbon dating of “old” charcoal using a wet oxidation, stepped-combustion procedure. Radiocarbon 41(2):127–140.
- Schmidt et al., Ref.13.
- Kirner et al., Ref. 68.
- Schleicher et al., Ref. 24.
- Schmidt et al., Ref. 13.
- Schmidt et al., Ref. 13.
- Schmidt et al., Ref. 13.
- Pearson, A., A. P. McNichol, R. J. Schneider, and C. F. Von Reden. 1998. Microscale AMS 14C measurements at NOSAMS. Radiocarbon 40:61–75.
- Nadeau et al., Ref. 14.
- Schmidt et al., Ref. 13.
- Beukens, R. P. 1993. Radiocarbon Accelerator Mass Spectrometry: Background and contamination. Nuclear Instruments and Methods in Physics Research B 79:620–623.
- Nadeau et al., Ref. 14.
- Bird et al., Ref. 89.
- Vogel, Nelson, and Southon, Ref. 21.
- Beukens, Ref. 11.
- Austin, S. A., J. R. Baumgardner, D. R. Humphreys, A. A. Snelling, L. Vardiman, and K. P. Wise. 1994. Catastrophic plate tectonics: A global Flood model of earth history. In, Walsh, R. E. (ed.). Proceedings of the third international conference on creationism, pp. 609–621. Pittsburgh, Pennsylvania: Creation Science Fellowship.
- Baumgardner, J. R. 1994a. Computer modeling of the large-scale tectonics associated with the Genesis Flood. In, Walsh, R. E. (ed.). Proceedings of the third international conference on creationism, pp. 49–62. Pittsburgh, Pennsylvania: Creation Science Fellowship.
- Baumgardner, J. R. 1994b. Runaway subduction as the driving mechanism for the Genesis Flood. In, Walsh, R. E. (ed.). Proceedings of the third international conference on creationism, pp. 63–75. Pittsburgh, Pennsylvania: Creation Science Fellowship.
- Baumgardner, J. R. 2003. Catastrophic plate tectonics: The physics behind the Genesis Flood. In, Ivey, R. L. (ed.). Proceedings of the fifth international conference on creationism, pp. 113–126. Pittsburgh, Pennsylvania: Creation Science Fellowship.
- Austin et al., Ref. 104.
- Baumgardner, Ref. 105.
- Baumgardner, Ref. 106.
- Baumgardner, Ref. 107.
- Baumgardner, Ref. 105.
- Baumgardner, Ref. 106.
- Baumgardner, Ref. 107.
- Brown, R. H. 1979. The interpretation of C-14 dates. Origins 6:30–44.
- Giem, Ref. 1.
- Scharpenseel, H. W. and P. Becker-Heidmann. 1992. Twenty-five years of radiocarbon dating soils: Paradigm of erring and learning. Radiocarbon 34:541–549.
- Nadeau et al., Ref. 14.
- Baumgardner, J. R. 2000. Distribution of radioactive isotopes in the earth. In, Vardiman, L., A. A. Snelling, and E. F. Chaffin (eds.), Radioisotopes and the age of the earth: A young-earth creationist research initiative, pp. 49–94. El Cajon, California: Institute for Creation Research and St. Joseph, Missouri: Creation Research Society.
- Humphreys, D. R., J. R. Baumgardner, S. A. Austin, and A. A. Snelling. 2003. Helium diffusion rates support accelerated nuclear decay. In, Ivey, R. L. (ed.). Proceedings of the fifth international conference on creationism, pp. 175–195. Pittsburgh, Pennsylvania: Creation Science Fellowship.
- Snelling, A. A. and M. H. Armitage. 2003. Radiohalos—A tale of three granitic plutons. In, Ivey, R. L. (ed.). Proceedings of the fifth international conference on creationism, pp. 243–267. Pittsburgh, Pennsylvania: Creation Science Fellowship.
- Baumgardner, Ref. 119.
- Humphreys et al., Ref. 120.
- Snelling and Armitage, Ref. 121.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


