Helium in Radioactive Rocks
Best Evidences from Science That Confirm a Young Earth
During the radioactive decay of uranium and thorium contained in rocks, lots of helium is produced. Because helium is the second lightest element and a noble gas—meaning it does not combine with other atoms—it readily diffuses (leaks) out and eventually escapes into the atmosphere. Helium diffuses so rapidly that all the helium should have leaked out in less than 100,000 years. So why are these rocks still full of helium atoms?
While drilling deep Precambrian (pre-Flood) granitic rocks in New Mexico, geologists extracted samples of zircon (zirconium silicate) crystals from different depths. The crystals contained not only uranium but also large amounts of helium.1 The hotter the rocks, the faster the helium should escape, so researchers were surprised to find that the deepest, and therefore hottest, zircons (at 387°F or 197°C) contained far more helium than expected. Up to 58% of the helium that the uranium could have ever generated was still present in the crystals.
The helium leakage rate has been determined in several experiments.2 All measurements are in agreement. Helium diffuses so rapidly that all the helium in these zircon crystals should have leaked out in less than 100,000 years. The fact that so much helium is still there means they cannot be 1.5 billion years old, as uranium-lead dating suggests. Indeed, using the measured rate of helium diffusion, these pre-Flood rocks have an average “diffusion age” of only 6,000 (± 2,000) years.3
These experimentally determined and repeatable results, based on the well-understood physical process of diffusion, thus emphatically demonstrate that these zircons are only a few thousand years old. The supposed 1.5-billion-year age is based on the unverifiable assumptions of radioisotope dating that are radically wrong.4
Another evidence of a young earth is the low amount of helium in the atmosphere. The leakage rate of helium gas into the atmosphere has been measured.5 Even though some helium escapes into outer space, the amount still present is not nearly enough if the earth is over 4.5 billion years old.6 In fact, if we assume no helium was in the original atmosphere, all the helium would have accumulated in only 1.8 million years even from an evolutionary standpoint.7 But when the catastrophic Flood upheaval is factored in, which rapidly released huge amounts of helium into the atmosphere, it could have accumulated in only 6,000 years.8
Rescuing Devices
So glaring and devastating is the surprisingly large amount of helium that old-earth advocates have attempted to discredit this evidence.
One critic suggested the helium didn’t all come from uranium decay in the zircon crystals but that a lot diffused into them from the surrounding minerals. But this proposal ignores measurements showing that less helium gas is in the surrounding minerals. Due to the well-established diffusion law of physics, gases always diffuse from areas of higher concentration to surrounding areas of lower concentration.9
Another critic suggested the edges of the zircon crystals must have stopped the helium from leaking out, effectively “bottling” the helium within the zircons. However, this postulation has also been easily refuted because the zircon crystals are wedged between flat mica sheets, not wrapped in them, so that helium could easily flow between the sheets unrestricted.10 All other critics have been answered.11 Thus all available evidence confirms that the true age of these zircons and their host granitic rock is only 6,000 (± 2,000) years.
Helium in Radioactive Rocks
Quick Escape of Helium
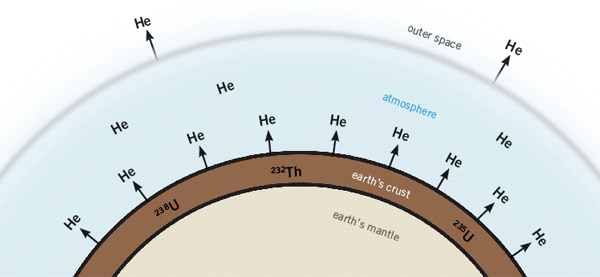
Figure 1: Radioactive elements in rocks produce a lot of helium as they decay. This gas quickly slips away into the atmosphere, especially when the rocks are hot. Yet radioactive rocks in the earth’s crust contain a lot of helium. The only possible explanation: the helium hasn’t had time to escape!
Answers Magazine
October – December 2012
God’s Word clearly teaches that the earth is young, and the evidence powerfully confirms it. So don’t miss this issue of Answers, which brings you up to speed on the ten best evidences for a young earth. Also discover incredible new examples of the Creator’s undeniable designs, a biblical view on political activism, the latest findings on the Dead Sea scrolls, and much more!
Browse IssueFootnotes
- R. V. Gentry, G. L. Glish, and E. H. McBay, “Differential Helium Retention in Zircons: Implications for Nuclear Waste Containment,” Geophysical Research Letters 9, no. 10 (1982): 1129–1130.
- S.
W. Reiners, K. A. Farley, and H. J. Hicks, “He Diffusion and (U-Th)/He Thermochronometry
of Zircon: Initial Results from Fish Canyon Tuff and Gold Butte, Nevada,” Tectonophysics
349, no. 1–4 (2002): 297–308;
D. Russell Humphreys, et al., “Helium Diffusion Rates Support Accelerated Nuclear Decay,” in Proceedings of the Fifth International Conference on Creationism, R. L. Ivey, Jr. (Pittsburgh, PA: Creation Science Fellowship, 2003), ed., pp. 175–196;
D. Russell Humphreys, “Young Helium Diffusion Age of Zircons Supports Accelerated Nuclear Decay,” in Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative, L. Vardiman, A.A. Snelling and E. F. Chaffin, eds. (El Cajon, CA: Institute for Creation Research, and Chino Valley, AZ: Creation Research Society, 2005), pp. 25–100. - Humphreys
et al., 2003;
Humphreys, 2005. - Andrew
A. Snelling, “Radiometric dating: Back to Basics,” Answers 4, no. 3 (July–Sept.
2009): 72–75;
Andrew A. Snelling, “Radiometric Dating: Problems With the Assumptions,” Answers 4, no. 4 (Oct.–Dec. 2009): 70–73. - G. E. Hutchinson, “Marginalia,” American Scientist 35 (1947): 118; Melvin A. Cook, “Where Is the Earth’s Radiogenic Helium?” Nature 179, no. 4557 (1957): 213.
- J. C. G. Walker, Evolution of the Atmosphere (London: Macmillan,
1977);
J. W. Chamberlain and D.M. Hunten, Theory of Planetary Atmospheres, 2nd edition (London: Academic Press, 1987). - Larry Vardiman, The Age of the Earth’s Atmosphere: A Study of the Helium Flux Through the Atmosphere (El Cajon, CA: Institute for Creation Research, 1990).
- For a fuller treatment and further information see:
John D. Morris, The Young Earth (Green Forest, AR: Master Books, 2000), pp. 83–85.
Don B. DeYoung, Thousands . . . Not Billions (Green Forest, AR: Master Books, 2005), pp. 65–78.
Andrew A. Snelling, Earth’s Catastrophic Past: Geology, Creation and the Flood (Dallas, TX: Institute for Creation Research, 2009), pp. 887–890. - D. Russell Humphreys, et al., “Helium Diffusion Age of 6,000 Years Supports Accelerated Nuclear Decay,” Creation Research Society Quarterly 41, no. 1 (2004): 1–16.
- Humphreys, 2005.
- D. Russell Humphreys, “Critics
of Helium Evidence for a Young World Now Seem Silent,” Journal of Creation
24, no. 1 (2010): 14–16;
D. Russell Humphreys, “Critics of Helium Evidence for a Young World Now Seem Silent?” Journal of Creation 24, no. 3 (2010): 35–39.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis