The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
An ongoing debate about the baraminic status of H. naledi has taken place in a series of recent articles in the creationist literature, which was first determined to be a part of the human holobaramin. Subsequent analyses have tried both to refute and support this idea. As a response to my previous rebuttal to the critique of my previous work suggesting that H. naledi is not a part of the human holobaramin, two papers have been presented arguing that the H. naledi remains could not have been deposited in the Dinaledi Chamber due to the Genesis Flood, but were intentionally buried, that the fossils were not mixed, that the cranial capacity of H. naledi falls within that of human variation, and that an analysis of cranial characters from 24 species including H. naledi show that it is part of the human holobaramin. On closer examination, the argument for intentional burial and homogeneity of fossils does not hold up. The cave system is much too convoluted and narrow to support regular burials of H. naledi individuals when burial in more easily accessible parts of the cave was possible. The three H. naledi skulls are also different in shape, and thus heterogenous. Compared to other known burial sites of modern humans, both remains of macrofauna and human artifacts are missing from the Dinaledi Chamber. Remains in these other sites are much more complete and articulated. H. floresiensis might well be another example of a fossil ape, based on both cranial and post-cranial characteristics (similar to H. naledi), mistaken to be a diminutive archaic human. Therefore, the cranial capacity of H. naledi still remains outside the range of modern humans. Baraminic analyses of one cranial data set and two post-cranial data sets suggest that both H. naledi and H. floresiensis are not part of the human holobaramin, but are rather australopiths.
Keywords: Homo naledi, baraminology, intentional burial, cranium, post-cranium, statistics
Intentional Burial?
In response to my first rebuttal (O’Micks 2016a), McLain (2017) claims that Homo naledi has to have been intentionally buried in the Dinaledi Chamber, because of several lines of evidence that it could not have been deposited by the Genesis Flood. However, just because these fossil remains were not deposited by the Genesis Flood, it doesn’t necessarily follow logically that they could only have been buried intentionally.
McLain (2017) still has not yet addressed certain facts which would make it hard to believe that the H. naledi remains would have been intentionally buried at that site. For example, the tunnel system leading from the surface to the Dinaledi Chamber is highly convoluted, and only 20 cm (7.8 in) across at some points, making it hard to carry fire along with the corpse of H. naledi. It took researchers two hours to get to the chamber, and they even had to climb ten meters up a large rock to get into the chamber. Why would these creatures intentionally bury their dead in a very hard to reach part of the cave system, every single time that a member of the group died? Why not bury the dead in a more easily accessible part of the cave with more light and which is much more easy to navigate?
In comparison, remains in the Qafzeh Cave in Israel show signs of modern burial behavior. First of all, the cave system is not described as being particularly hard to reach. Secondly, a number of stone tools, hearths, flint artifacts, and a collection of sea shells, with evidence of being strung together were found (Bar-Yosef Mayer 2009). No such objects were found in the Dinaledi Chamber, nor does it contain any faunal material (Laird et al. 2016). Another burial ground, Sima de los Huesos (Pit of the Bones in Spanish) in the Sierra de Atapuerca in Spain also contained material from larger bodied mammals, and also contained bones from the hand, feet, and spine which could be articulated with each other (Stringer 2015). Even though the remains of at least 28 individuals from this site were fragmented and scattered, they were well preserved (Carretero et al. 2012); 27 of them were virtually complete, as opposed to the H. naledi remains.
Furthermore, McLain (2017) writes: “In addition, O’Micks was incorrect in stating that humans do not tend to bury their dead alongside animal remains. This is demonstrated by the large number of such cases in the archaeological record, from Egyptians mummifying animals alongside their pharaohs to Iberians burying their dead with animal grave goods.”
Indeed, McLain (2017) is correct in that humans may bury their dead along with ornaments made from animal parts, such as antlers or teeth (which are missing from the Dinaledi chamber), but this is not what I was referring to in my first rebuttal; humans do not tend to bury their dead along with whole animal carcasses in the same grave. This is because humans would desire to be buried along with their own kind. This can be seen in the case of how Jacob wished to be buried along with his own people, his fathers, Abraham and Isaac in the cave of Machpelah in the land of Canaan (Genesis 49:28–33).
Bone Fragmentation
McLain (2017) also claims the following about the state of the H. naledi fossils: “O’Micks stated that the percentage of bone survival and fragmentation patterns of the bones are not what would be expected given a burial. These points are repeated from Val (2016). Dirks et al. (2016) addressed these issues by noting that most of the deposit is still unexcavated, so it is difficult to make an assessment as to the true percentage of bone survival.”
We have to note that the H. naledi fossils had been found rather close to the surface, and that even according to McLain (2017) the fossil remains may be quite young, even post-Flood. This means that the H. naledi remains could certainly have survived unfragmented for a relatively short amount of geological time. Furthermore, the claim that most of the deposit is still unexcavated is an argument from silence, it would be necessary to produce the remaining bones for the author to support his claim. The percent of bone survival is only 10.8% (Val 2016) which is very fragmented, and not what you would expect if the remains were intentionally buried. This also argues for possible carnivorous animals having chewed on the bones.
Mixed Fossils

Fig. 1. The right hand of H. naledi (MorphoSource ID M10173). Note the long, curved, australopithecine-like finger bones.
McLain (2017) also writes: “O’Micks suggested that H. naledi may be a chimaeric taxon, including bones from animals and humans. Given the difficulty for biogenic and abiogenic agents to add specimens to the chamber, as well as the lack of nonhominin macrovertebrate remains in the deposit, this proposal seems unlikely.” The fact remains that the fossils show characteristics of both humans and apes, namely the long, curved fingers (fig. 1), the distally wide rib cage, and the australopith-like shoulders.
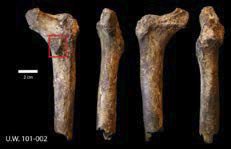
Fig. 2. MorphoSource photo of the proximal right femur of H. naledi (MorphoSource ID M6540, ID UW-101- 002). The bony bulge can be seen in the red box pointing backwards. The fossil is shown from the posterior, proximal, anterior, and distal viewpoints.
Wood (2017) asks “If there were two different hominin species preserved in the Dinaledi chamber, why do we not find a single example of two bones of the same type that are markedly different?” Dr. Jeffrey Schwartz had his opinions also published in Nature News (Callaway 2015), and the fact still remains that two of the H. naledi skulls are long and low, with sloping foreheads, whereas one is short and rounded. Furthermore, according to Schwartz, in australopiths, there is a small bump of bone below the neck of the femur, which points backwards. In contrast, this bump points inwards in humans. In Fig. 2 we can see that in H. naledi the bump points backwards, suggesting that it is an australopith. Therefore, I still maintain that the H. naledi remains could possibly come from different taxa.
Statistical Considerations and Post-Cranial Variation
Wood (2017) states in his paper that “In 2016, two statistical baraminology studies identified Homo naledi as part of the human holobaramin (O’Micks 2016b; Wood 2016a), but in a subsequent analysis of postcranial characters, O’Micks (2016b) reversed his assessment and concluded that Homo naledi is not human after all. His analysis of postcranial characters focused on only a handful of taxa, and in a response, Wood (2016b) showed that his results were a statistical artifact of his small sample size. O’Micks (2016c) then conceded that Wood’s critique was valid but continued to maintain that Homo naledi is not human.”
It is not true that I conceded that my re-analysis of H. naledi was a statistical artifact, Wood (2017) overlooks the fact that different weighting factors were used during character transformation in both the original analysis of H. naledi as well as the reanalysis done by O’Micks, which may possibly be the reason for the changes in the baraminic distance correlations. Furthermore, Wood (2016b) also makes the statement that: “O’Micks also introduced a novel weighting scheme to give 62 craniodental and 37 postcranial characters equal weight when calculating baraminic distances. Although baraminic distances are traditionally calculated in an unweighted scheme to avoid researcher bias, the weighting scheme seems justified in this instance, given the importance of postcranial characters” (emphasis added).
This is important, because here Wood (2016b) recognizes the importance of weighting different morphological characteristics based on the intuitive value of a given character. Thus, this is a far cry from “data manipulation” as Wood (2017) stated. For example, cranial volume is a more significant character, since it is reflective of intelligence and mental capacity, as opposed to measuring the height, width, and breadth of a single tooth or other bone structure which is redundant and does not provide too much extra information. Furthermore, craniodental characteristics could be given a smaller weight than postcranial characters, since the cranium is proportionally smaller than the post-cranium, and also more craniodental characters were included in my reanalysis of H. naledi (62) compared to the number of postcranial characters (37). Therefore, the second claim by Wood (2017) is not true that “In other words, as has long been known in systematics, certain characters appear to be more useful for distinguishing groups of organisms than other characters. Once again, the challenge here is justifying which characters ought to be favored, which O’Micks does not do”. At least a couple of suggestions were outlined here and also in my re-analysis of H. naledi.
Indeed, if we look at Figs. 5a–e in O’Micks (2016b) we see that baraminic distance tends to decrease between H. naledi and the three Australopithecus species being studied and also tends to increase between H. naledi and the two Homo species, as the relevance cutoff increases and the weighting factor decreases. Opposite trends can be observed for baraminic correlation, seen in Figs. 6a–e of O’Micks (2016b). Though the baraminic correlations may not be significant, these trends hint at the fact that H. naledi is really not part of the human holobaramin, but is rather an australopith.
Wood (2017) also states the following: “Indeed, given biblical references to giants, one might suppose that postcranial variations should be expected. This is not to say that the Bible teaches that humans once possessed postcranial characters found in australopiths, as O’Micks (2016c) mistakenly inferred, but that the previous existence of giants shows us that postcranial skeletal characteristics probably varied and cautions against inappropriately favoring them as a means of recognizing humans in the fossil record.”
I have to reiterate the fact that nowhere does the Bible mention that these giants showed any kind of variation in their postcranial skeleton (other than obviously their size), resembling australopiths or any other kind of organism. That “postcranial skeletal characteristics probably varied” is purely a speculation of Wood (2017), and it is incumbent upon him to show any Bible verse which says so, or to produce the postcranial skeletons of these Old Testament giants, which show any kind of postcranial variation. Otherwise, this is merely an argument from silence.
Cranial Capacity
The cranial capacity of H. naledi was measured to be between 465 and 560 cc. Wood (2017) makes an attempt to close the gap between H. naledi and humans by citing cranial capacities of several fossil humans, such as eight H. erectus crania between 600 and 800 cc. Wood (2017) mentions the cranial volume of H. habilis as being between 500 and 600 cc. However, H. habilis has been rejected as being human by most creationists and even some evolutionists (Lubenow 2004; O’Micks 2016a). Interestingly, Wood (2017) mentions the cranial capacity of H. floresiensis, which is 380–417 cc (Falk et al. 2005; Lahr and Foley 2004), and also which some creationists accept as human.
This argument is disputable, since it is well likely that H. floresiensis is also not human. Thus, since the cranial capacity of H. naledi remains outside of the human range, there is no convincing fact that it is human. Indeed, if H. floresiensis is truly human, one could wonder why no intermediary fossils have been found between it and other fossil humans, or between other humans and H. naledi, for that matter. Lahr and Foley (2004) state that the relative brain and body size of H. floresiensis are outside not only the human range, but even also the range of australopithecines!
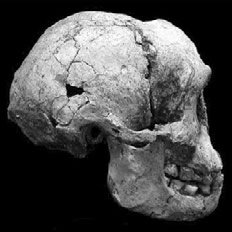
Fig. 3. Side view of the LB1 skull (H. floresiensis), taken from Brown et al. 2004.
Furthermore, H. floresiensis has bony reinforcements alongside its nose (which is lacking in humans), and also thigh bones which are less obliquely aligned than those of humans. The oblique thigh bones are a crucial postcranial character, since the alignment of the thigh bones influences gait and body posture, making it possible that H. floresiensis wasn’t even bipedal. The pelvic bones of H. floresiensis were also very wide, which influences body shape, and which is also characteristic of apes (Park 2012). Furthermore, when viewing the skull of the LB1 specimen, one can see that it lacks a nasal bone, has a slightly sloping face, and forwardlooking eye sockets, which are characteristic of apes (see fig. 3). The crown and root morphology of the teeth are also similar to those of australopithecines (Brown et al. 2004). Morwood et al. (2004) also record a humerofemoral index of 84.5 for LB1, which is outside the range for H. sapiens, but which matches with that of A.L. 288-1 (A. afarensis).
Therefore, Wood (2017) cannot argue that the cranial capacity gap between H. naledi and humans has been bridged by Homo floresiensis, which could be an australopith instead.
Reanalysis of Post-Cranial Characteristics of H. naledi
Measurements for 14 tibial characteristics for seven species (H. sapiens, H. erectus, early Homo, H. naledi, Australopithecus sp., G. gorilla, and P. troglodytes) were analyzed, which were described in Table 5 of Marchi et al. (2016). Baraminic distance correlation (BDC) and 3D multidimensional scaling (MDS) were calculated using BDISTMDS (http://www.coresci.org/bdist.html) (Robinson and Cavanaugh 1998; Wood 2005, 2008). Ten of the 14 characters had a relevance cutoff > 0.95.
Two small clusters were formed, 1) one with the two human species, H. sapiens and H. erectus, and 2) one with three species, H. naledi, Australopithecus sp., and early Homo, P. troglodytes, and G. gorilla were isolated from these two small groups (figs 4 and 5). What is significant is that there is a significant positive BDC between H. sapiens and H. erectus, and a significant negative BDC between these two species and the three members of the second cluster (which contains H. naledi) as well as early Homo. The baraminic distance between the members of the first cluster is quite low, 0.1 (with a bootstrap value of 100), as is the average baraminic distance between the members of the second cluster (0.3). A bootstrap value of 98 exists between H. naledi and Australopithecus sp.
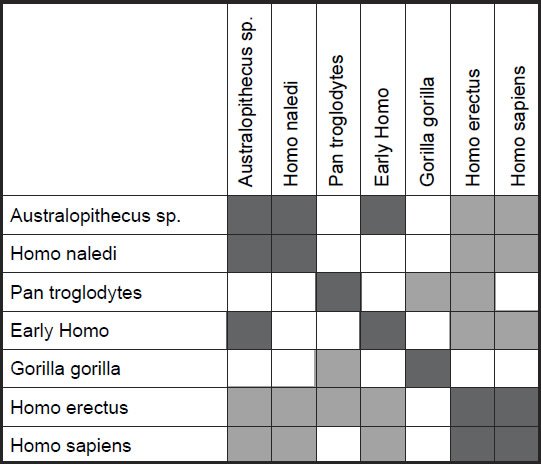
Fig. 4. Baraminic distance correlation results for measurements on the tibia, taken from Table 5 of Marchi et al. (2016). Dark gray boxes denote significant, positive BDC, whereas light gray squares denote significant, negative BDC.
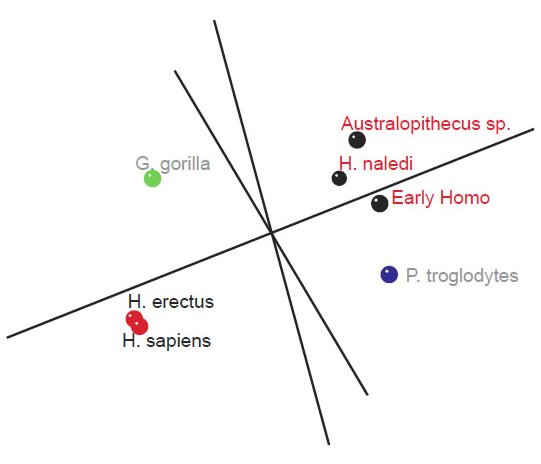
Fig. 5. Three-dimensional MDS results for measurements on the tibia, taken from Table 5 of Marchi et al. (2016). A first cluster of species can be seen in black (Early Homo, H. naledi, and Australopithecus sp.). A second group of species can be seen in red (H. sapiens and H. erectus). P. troglodytes is shown in blue, and G. gorilla in green.
As for the MDS results, H. sapiens + H. erectus cluster together, as do early Homo + H. naledi + Australopithecus sp (fig. 5). There is a minimal stress value of 0.054 at three dimensions. Measurements for 18 characters of the scapula, clavicle, humerus, ulna, and radius were described in Table 2 of Feuerriegel et al. (2016) for seven species (A. sediba, A. africanus, A. afarensis, H. floresiensis, H. habilis, H. naledi, and H. erectus). BDC results were obtained at a relevance cutoff > 0.95 for five characters. Three pairs of species formed: 1) A. afarensis + A. sediba, 2) H. habilis + H. naledi, and 3) A. africanus + H. floresiensis (fig. 6). H. erectus did not show any positive BDC to any of these species, and showed significant negative BDC to the species in group #1. In the MDS results (fig. 7), A. sediba and A. afarensis have the same coordinates, which is an artifact of the small number of characters passing the relevance cutoff. There is a minimal stress of 0.178 at two dimensions.
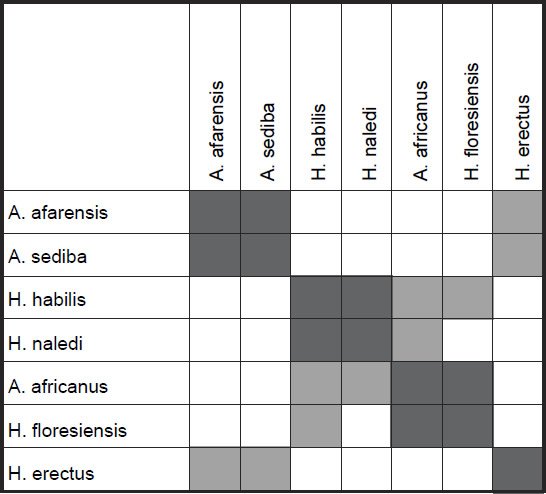
Fig. 6. Baraminic distance correlation results for measurements on the clavicle, scapula, humerus, ulna, and radius, taken from Table 2 of Feuerriegel et al. (2016). Dark gray boxes denote significant, positive BDC, whereas light gray squares denote significant, negative BDC.
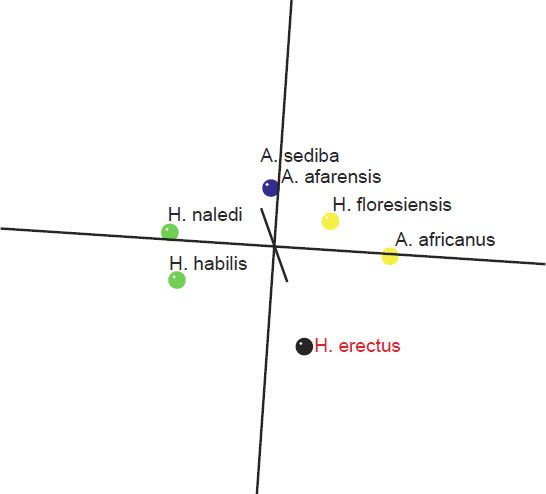
Fig. 7. Three-dimensional MDS results for measurements on the clavicle, scapula, humerus, ulna, and radius, taken from Table 2 of Feuerriegel et al. (2016). A. africanus and A. sediba can be seen in blue, on top of each other due to an artifact of too few characters passing the relevance cutoff. A. africanus and H. floresiensis can be seen in yellow in another group. H. naledi and H. habilis form a third pair, seen in green, and H. erectus is in black, separate from the previous three pairs.
What is important to notice here in these results is that for one, A. sediba groups with A. afarensis, thereby contradicting the results of Wood (2010), who put A. sediba into the human holobaramin. Furthermore, H. floresiensis is paired with A. africanus, thereby further supporting the idea detailed in this paper that H. floresiensis is an australopith, contradicting the second author’s claim that it belongs to the human holobaramin. Also, H. naledi groups together with H. habilis, which, as stated earlier is disputed by some to be a mixed taxon (Lubenow 2004).
An analysis of a third data set of 14 cranial characteristics described in Table 3 of Laird et al. (2016) was also performed for seven species (A. africanus, A. sediba, H. nabilis, H. naledi, H. rudolfensis, H. erectus, and H. sapiens). This data set is interesting, in that it is a new data set, not just a modified version of Berger at al.’s (2015) data set. H. habilis groups together with A. africanus, suggesting that it is not a part of the human holobaramin. H. erectus shows significant negative BDC with these two species (fig. 8). There is a minimal stress of 0.067 at three dimensions. The MDS results (fig. 9) show A. africanus + H. habilis and the other species apart from each other. Otherwise, the results are inconclusive, but they do not support definitively placing H. naledi into the human holobaramin.

Fig. 8. Baraminic distance correlation results for skull measurements taken from Table 3 of Laird et al. (2016). Dark gray boxes denote significant, positive BDC, whereas light gray squares denote significant, negative BDC.
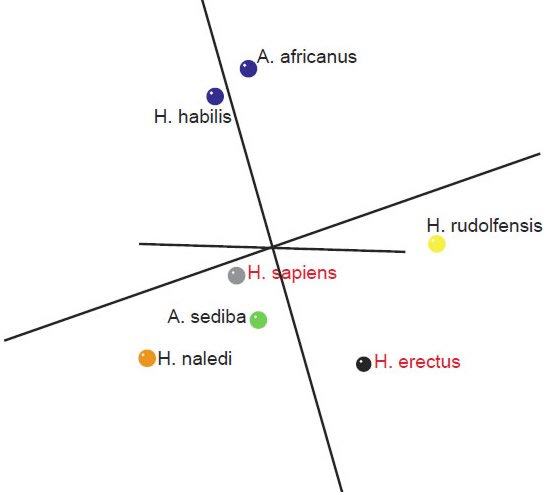
Fig. 9. Three-dimensional MDS results for skull measurements taken from Table 3 of Laird et al. (2016). Blue: A. africanus and H. habilis. Green: A. sediba. Yellow: H. rudolfensis. Orange: H. naledi. Gray: H. sapiens. Black: H. erectus.
The character data and the results of the BDIST analysis for all three of these data sets can be found in the supplementary data file.
Summary and Conclusion
In summary, McLain (2017) and Wood (2017) try to portray H. naledi as a clear-cut case of being human, despite many evidences to the contrary. For example, the question of how and why H. naledi would bury its dead in such a hard to reach part of the cave is left unaddressed. The fact that the shape of the three H. naledi skulls is different (round versus long and narrow) was not addressed; the shape of the skull is a very important factor in identifying a species, since sometimes this is the only fossil that paleontologists can go by, as in the case of Java man (Lubenow 2004). The H. naledi remains are very fragmented and are unaccompanied by the remains of macrofauna, artificial tools or ornaments, as in the case of other identified burial sites of modern humans. Furthermore, the authors erroneously claim that since the cranial capacity of H. floresiensis is smaller than that of H. naledi, and since H. floresienesis is accepted by some as human, therefore H. naledi must also be human (even though the cranial capacity of H. floresienesis itself is outside of the range of australopithecines). Both H. naledi and H. floresiensis show quite a few australopith-like characteristics, therefore they may be similar cases. No intermediate fossils have been shown to traverse the gap between larger-sized humans and the fossils of the diminutive H. naledi and H. floresiensis. In fact, the lack of fossil intermediates between larger-sized humans and these two species is a sign of baraminic discontinuity separating them from the human holobaramin.
I disagree with the statement by Wood (2017) that “Surprisingly, O’Micks (2016c) also disagreed with the statement, ‘all humans, no matter their appearance, are descendants of Adam and Eve’.” All humans are descendants of Adam and Eve, there is no doubt about that. What I did mean to say is that it might not be correct to keep on lumping newer and newer species into the human holobaramin, even going so far as to lump something which is not human into the human holobaramin. That is why we must beware of one hand clapping, as the saying goes, and rely on multiple lines of evidence to get a more holistic view of the baraminic status of a given species. Practical knowledge of anatomy and the importance of different characters must be taken into account as a reality check. Therefore, it might be possible that lumping H. naledi into the human holobaramin, along with A. sediba itself might be a statistical artifact, which might be alleviated with a weighting scheme for the different characters.
The analysis by Wood (2017) of skull characters from a recently published character matrix (Dembo et al. 2016) may seemingly show that both A. sediba and H. naledi group with the human holobaramin. However, the present results for bones from the upper and lower limbs contradict these results. This illustrates the need for a holistic analysis of both cranial and postcranial characters together. Determining the baraminic status of fossil humans can be difficult. While it is true that we should not force our preconceived notions of what humans are onto the evidence, still, human baraminology is unique in certain ways. Homo sapiens sapiens, or modern human, is the only remaining variant of the human holobaramin existing today, so therefore the human status of other species must be inferred in relation to ourselves (modern humans). Inferring relatedness to fossil species is made difficult by the fragmented state of these fossils as well as the lack of soft tissue. Hybridization between different variants of the human holobaramin is also impossible, which is a key method of baraminology (Wood and Murray 2003), although it is possible with modern sequencing technology to detect genetic mixing between modern and archaic humans such as Neanderthals and Denisovans (Savanne 2014). Indeed, it would be very much useful if the whole genome sequences of all archaic humans could be determined, as well as for H. naledi.
Materials and Methods
Figs. 1 and 2 were taken from the MorphoSource Database at Duke University (http://morphosource.org/). Fig. 3 was taken from Brown et al. (2004). The character scores from Table 2 of Feuerriegel et al. 2016 and Table 5 of Marchi et al. 2016 were both transformed according to the description in O’Micks (2016a), with a scaling factor of 3.999. A scaling factor of 2.999 was used to analyze the data from Laird et al. (2016). Kinemage software was used to visualize the MDS results (available at http://kinemage.biochem.duke.edu/software/mage.php).
References
Bar-Yosef Mayer D. E., B. Vandermeersch, and O. Bar-Yosef. 2009. “Shells and Ochre in Middle Paleolithic Qafzeh Cave, Israel: Indications for Modern Behavior.” Journal of Human Evolution 56 (3): 307–314.
Berger, L. R., J. Hawks, D. J. de Ruiter, S. E. Churchill, P. Schmid, L. K. Delezene, T. L. Kivell, et al. 2015. “Homo naledi, a New Species of the Genus Homo from the Dinaledi Chamber, South Africa.” eLife 4: e09560.
Brown, P., T. Sutikna, M. J. Morwood, R. P. Soejono, Jatmiko, E. W. Saptomo, and R. A. Due. 2004. “A New Small-Bodied Hominin From the Late Pleistocene of Flores, Indonesia.” Nature 431 (7012): 1055–1061.
Callaway E. 2015. “Crowdsourcing Digs Up an Early Human Species.” Nature 525 (7569): 297–298.
Carretero, J.-M., L. Rodríguez, R. García-González, J.-L. Arsuaga, A., Gómez-Olivencia, C. Lorenzo, A. Bonmati, A. Gracia, I. Martinez, and R. Quarm. A. 2012. “Stature Estimation From Complete Long Bones in the Middle Pleistocene Humans From the Sima de los Huesos, Sierra de Atapuerca (Spain).” Journal of Human Evolution 62 (2): 242–255.
Dembo, M., D. Radovčić, H. M. Garvin, M. F. Laird, L. Schroeder, J. E. Scott, J. Brophy, et al. 2016. “The Evolutionary Relationships and Age of Homo naledi: An Assessment Using Dated Bayesian Phylogenetic Methods.” Journal of Human Evolution 97: 17–26.
Dirks, P. H. G. M., L. R. Berger, J. Hawks, P. S. Randolph-Quinney, L. R. Backwell, and E. M. Roberts. 2016. “Comment on ‘Deliberate Body Disposal by Hominins in the Dinaledi Chamber, Cradle of Humankind, South Africa?’” Journal of Human Evolution 96: 149–153.
Falk, D., C. Hildebolt, K. Smith, M. J. Morwood, T. Sutikna, P. Brown, Jatmiko, E. W. Saptomo, B. Brunsden, and F. Prior. 2005. “The Brain of LB1, Homo floresiensis.” Science 308 (5719): 242–245.
Feuerriegel, E. M., D. J. Green, C. S. Walker, P. Schmid, J. Hawks, L. R. Berger, and S. E. Churchill. 2016. “The Upper Limb of Homo naledi.” Journal of Human Evolution. doi.org/10.1016/j.jhevol.2016.09.013.
Lahr, M. M., and R. Foley. 2004. “Palaeoanthropology: Human Evolution Writ Small.” Nature 431 (7012): 1043–1044.
Laird, M. F., L. Schroeder, H. M. Garvin, J. E. Scott, M. Dembo, D. Radovčić, C. M. Musiba, et al. 2016. “The Skull of Homo naledi.” Journal of Human Evolution. doi.org/10.1016/j.jhevol.2016.09.009.
Lubenow, M. L. 2004. Bones of Contention: A Creationist Assessment of Human Fossils. Grand Rapids, Michigan: Baker Books.
Marchi, D., C. S. Walker, P. Wei, T. W. Holliday, S. E. Churchill, L. R. Berger, and J. M. DeSilva. 2016. “The Thigh and Leg of Homo naledi.” Journal of Human Evolution. doi.org/10.1016/j.jhevol.2016.09.005.
McLain, M. 2017. “Reply to O’Micks Concerning the Geology and Taphonomy of the Homo naledi Site.” Answers Research Journal 10: 55–56.
Morwood, M. J., R. P. Soejono, R. G. Roberts, T. Sutikna, C. S. M. Turney, K. E. Westaway, W. J. Rink, et al. 2004. “Archaeology and Age of a New Hominin From Flores in Eastern Indonesia.” Nature 431 (7021): 1087–1091.
O’Micks, J. 2016a. “Preliminary Baraminological Analysis of Homo naledi and Its Place Within the Human Baramin.” Journal of Creation Theology and Science Series B: Life Sciences 6: 31–39.
O’Micks, J. 2016b. “Homo naledi Probably Not Part of the Human Holobaramin Based on Baraminic Re-Analysis Including Postcranial Evidence.” Answers Research Journal 9: 263–272.
O’Micks, J. 2016c. “Reply to ‘Taxon Sample in Hominin Baraminology: A Response to O’Micks’.” Answers Research Journal 9: 373–375.
Park, M. A. 2012. Biological Anthropology. 6th ed. New York: McGraw-Hill Companies, Inc.
Robinson, D. A., and D. P. Cavanaugh. 1998. “A Quantitative Approach to Baraminology with Examples From the Catarrhine Primates. Creation Research Society Quarterly 34 (4): 196–208.
Savanne, D. 2014. “Denisovans Menace Evolution—A New Chapter in the Human Origins Debate.” Journal of Creation 28 (3): 5–8.
Stringer C. 2015. “The Many Mysteries of Homo naledi.” eLife 4: 10627.
Val, A. 2016. “Deliberate Body Disposal by Hominins in the Dinaledi Chamber, Cradle of Humankind, South Africa?” Journal of Human Evolution 96: 145–148.
Wood, T. C., and M. J. Murray. 2003. Understanding the Pattern of Life: Origins and Organization of the Species. Nashville, Tennessee: Broadman & Holman.
Wood, T. C. 2005. “Visualizing Baraminic Distances Using Classical Multidimensional Scaling.” Origins (GRI) 57: 9–29.
Wood, T. C. 2008. BDISTMDS software, v. 2.0. Dayton, Tennessee: Center for Origins Research, Bryan College.
Wood, T. C. 2010. “Baraminological Analysis Places Homo habilis, Homo rudolfensis, and Australopithecus sediba in the Human Holobaramin.” Answers Research Journal 3: 71–90.
Wood, T. C. 2016a. “An Evaluation of Homo naledi and ‘Early’ Homo From a Young-Age Creationist Perspective.” Journal of Creation Theology and Science Series B: Life Sciences 6: 14–30.
Wood, T. C. 2016b. “Taxon Sample Size in Hominin Baraminology: A Response to O’Micks.” Answers Research Journal 9: 369–372.
Wood, T. C. 2017. “Identifying Humans in the Fossil Record: A Further Response to O’Micks.” Answers Research Journal 10: 57–62.
Supplementary Tables
Transformed data from Marchi et al., 2016, Feuerriegel et al., 2016, and Laird et al., 2016, along with results from BDIST program.










