The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
Recent articles have questioned the validity of hominin baraminology studies that place Homo naledi in the human holobaramin. Despite questions about the fossil discoveries, the skeletal remains of Homo naledi were intentionally placed within the cave chamber and represent a single species. Holistic considerations of burial behavior and statistical baraminology support recognizing Homo naledi as human, despite a smaller cranial capacity than modern humans. Theoretical concerns about morphological discontinuity as an indicator of holobaramins remain important questions for future study, but recent analyses reveal the presence discontinuity around the human holobaramin. New statistical baraminology analysis of a recently published character matrix continues to show a clear separation between a cluster of taxa that includes modern humans and other taxa clearly not human (e.g. chimpanzee). Consequently, worries about baraminology supporting the inclusion of nonhumans within the human holobaramin appear to be unwarranted.
Keywords: Homo naledi, statistical baraminology, hominin, hominid
In 2016, two statistical baraminology studies identified Homo naledi as part of the human holobaramin (O’Micks 2016a; Wood 2016a), but in a subsequent analysis of postcranial characters, O’Micks (2016b) reversed his assessment and concluded that Homo naledi is not human after all. His analysis of postcranial characters focused on only a handful of taxa, and in a response, Wood (2016b) showed that his results were a statistical artifact of his small sample size. O’Micks (2016c) then conceded that Wood’s critique was valid but continued to maintain that Homo naledi is not human.
O’Micks (2016c) raised six issues regarding statistical baraminology and the status of Homo naledi that warrant further comment. Perhaps most importantly, he questions whether Homo naledi represents a single species or a mix of animal and human remains. If correct, “Homo naledi” is not a valid category, and all discussion of its status would change substantially. O’Micks notes that Homo naledi has a substantially smaller cranial capacity than modern humans and even than Homo erectus. He also questions whether there might be holobaramins so similar that they cannot be distinguished without special weighting of characters. All of these objections follow his emphasis on analyzing holobaramins holistically rather than just using craniodental characters and statistical methodology. According to O’Micks, overreliance on statistics could lead to inappropriately placing animals in the human holobaramin. Each of these issues will be addressed here.
One Species?
O’Micks (2016c) questioned whether there might be more than one species’ remains in the Dinaledi chamber. O’Micks cited an opinion piece from Newsweek written by Jeffrey Schwartz (2015), wherein Schwartz claimed that the H. naledi fossils represent more than one species. In reality, the likelihood that these fossils represent two different species is incredibly remote. The 1550 bones from the Dinaledi chamber includes multiple samples of the same bone. For example, Marchi et al. (2016) record nine different left femoral elements, and Harcourt-Smith et al. (2015) record six examples of the left talus. Additional examples of multiple copies of each bone could be cited from further anatomical descriptions (Feuerriegel et al. 2016; Kivell et al. 2015; Laird et al. 2016). In all cases, the bones are extremely similar to each other, so similar that Wise (2016) speculated that the bones might have come from a single family. If there were two different hominin species preserved in the Dinaledi chamber, why do we not find a single example of two bones of the same type that are markedly different? If Schwartz (2015) has additional information about the bones that supports recognizing two different species, he should publish his own analysis in an appropriate forum other than a Newsweek editorial. The probability that Homo naledi represents a more than one species is so impossibly small that we may safely ignore it. Homo naledi bones come from a single species.
Cranial Capacity
O’Micks (2016c) also cited the small cranial capacity of Homo naledi (approximately 460 cc, outside the range of modern adult humans) in favor of excluding H. naledi from the human holobaramin. To this we may first refer to the small cranial capacity of the adult skull LB1 of Homo floresiensis, which has an estimated cranial capacity of only 417 cc (Falk et al. 2005) but which is recognized as human by multiple creationists (Line 2006; Tyler 2005; Wise 2005). Further, based on cranial capacities of fossils listed by Schoenemann (2013) and De Miguel and Henneberg (2001), there are eight Homo erectus crania with endocranial volumes ranging from 600 to 800 cc, substantially bridging the gap between H. floresiensis and fossils O’Micks would presumably accept as human. If we expand our sample of human taxa to include Homo habilis, as the baraminology results would suggest, three additional adult crania have endocranial volumes between 500 and 600 cc. Thus, even though the majority of modern and fossil humans have brains much larger than Homo naledi, H. naledi is not completely excluded from the range of known human cranial capacities.
Discontinuous Holobaramins?
O’Micks (2016c) also wondered whether morphological discontinuity should be considered a defining characteristic of holobaramins. In statistical baraminology, a holobaramin is a group of taxa that share continuity (significant, holistic similarity) and are separated from all other taxa by discontinuity (significant, holistic difference) (Wood et al. 2003). Whether or not this holobaramin concept is valid is a question well worth pondering, not least because so much reasoning in creationism proceeds on the assumption that discontinuity should be evident between humans and apes. In contrast, Wood (2011) proposed that discontinuity around holobaramins should be treated as a hypothesis. Statistical baraminology can then be considered a means of testing this “discontinuity hypothesis,” a test which thus far has failed to falsify the existence of morphological discontinuity around the human holobaramin. Contrary to public presentations of the hominin fossil record, we do not find humans insensibly grading into nonhumans using statistical baraminology. Statistical baraminology studies have shown that humans are humans, and nonhumans are nonhumans. There are no known intermediate forms that bridge the two groups.
Along this same line of thought, O’Micks (2016c) speculated that there could be two very similar holobaramins that would be difficult to distinguish using statistical baraminology. While that is certainly possible, it is not clear why O’Micks favors this scenario other than his motivation to exclude Homo naledi from the human holobaramin. In statistical baraminology, two clusters that are indistinguishable would be considered a single cluster and likely recognized as a holobaramin until such time as new characters or evidence call for a reappraisal.
Weighted Characters
Following his suggestion of two very similar created kinds, O’Micks (2016c) proposed that calculating baraminic distances using a weighting scheme could support excluding Homo naledi from the human holobaramin. He further claimed that we ought to distinguish between “more or less important morphological characteristics.” In other words, as has long been known in systematics, certain characters appear to be more useful for distinguishing groups of organisms than other characters. Once again, the challenge here is justifying which characters ought to be favored, which O’Micks does not do. Instead, he noted that selecting a certain weighting scheme would provide some statistical support for excluding Homo naledi from the human holobaramin. Selecting a methodology because it gives the desired outcome is more akin to data manipulation than data analysis. Any weighting scheme used in baraminology must be justified independently of the outcome before we can take it seriously.
It is true that creationists have traditionally favored postcranial characteristics for recognizing which hominins are human, but there is no a priori reason to suspect that human postcranial characters should be invariable and therefore unerringly signal which hominin fossils are truly human. Indeed, given biblical references to giants, one might suppose that postcranial variations should be expected. This is not to say that the Bible teaches that humans once possessed postcranial characters found in australopiths, as O’Micks (2016c) mistakenly inferred, but that the previous existence of giants shows us that postcranial skeletal characteristics probably varied and cautions against inappropriately favoring them as a means of recognizing humans in the fossil record.
Holistic Created Kinds
O’Micks (2016c) is undoubtedly correct that baraminology ought to study created kinds using a “holistic” approach that considers multiple lines of evidence. Indeed, the “refined baramin concept” of Wood et al. (2003), which forms the theoretical foundation for statistical baraminology, defines apobaramin, monobaramin, and holobaramin in holistic terms. Thus far, the most definitive statistical baraminology research on humans has focused on the craniodental characters favored by paleoanthropologists (Wood 2010, 2016a), which as O’Micks correctly notes is not very holistic. Efforts to include postcranial information in baraminological research on hominins is undoubtedly warranted and desirable, but efforts to include such information has thus far been inconclusive (Wood 2016b).
Can we say anything holistic about fossil hominins apart from statistical baraminology? With Neanderthals and Homo erectus, the evidence seems clear and compelling. Homo erectus is known to have made tools (e.g., Joordens et al. 2014) and used fire (Gowlett 2016; James 1989), two complex behavioral characteristics that seem very human. In addition, the postcranial material known from H. erectus (e.g. Nariokotome boy) is extremely similar to modern Homo sapiens. In the case of Neanderthals, the evidence seems even clearer. We find Neanderthal burials (e.g., Rendu et al. 2014), Neanderthal artwork (e.g., Radovčić et al. 2015; Welker et al. 2016; Zilhão et al. 2010), complex Neanderthal tools made of stone and bone (Soressi et al. 2013), in addition to a postcranial skeleton that is also extremely similar to modern humans. Most persuasive of all is the evidence from the Neanderthal genome studies, which have revealed ancient interbreeding between Neanderthals and the ancestors of modern Eurasians (Green et al. 2010). For both H. erectus and Neanderthals, a holistic consideration of all evidence points to the humanity of both groups of creatures.
The challenge then comes from taxa where the evidence is much less clear. Many of these taxa are classed under the evolutionary term “early Homo,” and most have much less fossil material associated with them. Whereas Neanderthals and H. erectus are known from many fossils throughout their geographic range, “early Homo” are very often known from comparatively few fossils from a much more limited geographic range (sometimes just a single site). Named taxa with very little fossil evidence include Homo rudolfensis and Homo habilis. Other taxa like A. sediba have substantially more fossil evidence but thus far are known from a single locality. Even within paleoanthropology, some experts disagree on species designations of certain discoveries (e.g., Wood and Boyle 2016). In these more ambiguous cases, statistical baraminology can provide a supplement to the qualitative evaluations used in the case of H. erectus or Neanderthals.
In this area of uncertainty in hominin baraminology, Homo naledi presents a special case. Even though H. naledi is currently known only from a single location, the Dinaledi chamber in the Rising Star Cave, more than 1500 bones have been recovered and untold thousands probably remain to be excavated (Berger et al. 2015). In addition, the unique features of the depositional environment strongly suggest that the remains were placed in the chamber intentionally by other, living Homo naledi (Dirks et al. 2015). In other words, the geological evidence supports the inference that Homo naledi practiced a form of intentional burial. The dissenting opinions cited by O’Micks (2016c) have all been satisfactorily answered by Dirks et al. (2016) and Randolph-Quinney et al. (2016). Other than intentional burial, there has been no proposal for the emplacement of H. naledi remains within the Dinaledi chamber that adequately explains the geological and taphonomic features of the site. Evidence of intentional burial does not constitute an advanced “religious” behavior, as O’Micks (2016c) mistakenly inferred, but rather a repetitive ritual. Why H. naledi engaged in this ritual can only be a matter of speculation, but the evidence clearly supports the inference that they did so.
Considered holistically then, the evidence of intentional burial, which seems so human, and the statistical baraminology results of Wood (2016a) and O’Micks (2016a) all support recognizing H. naledi as human, despite marked differences in the postcranial characters of H. naledi and modern humans. This is not simply a case of overreliance on a single statistical study, as O’Micks (2016c) asserted. In contrast, Homo naledi is perhaps one of the clearest cases of an identifiably human hominin outside of modern humans, Neanderthals, and Homo erectus.
Future Speculations
Surprisingly, O’Micks (2016c) also disagreed with the statement, “all humans, no matter their appearance, are descendants of Adam and Eve.” O’Micks explained his objection to that statement by speculating that a statistical baraminology study might one day show evidence of continuity between humans and nonhumans. Why such a scenario would invalidate the descent of all humans from Adam and Eve is unclear, but more importantly, the vulnerability of the discontinuity hypothesis to refutation by statistical baraminology is one of the most important features that makes baraminology scientific. If we merely approach the hominin fossil record with a preconceived notion of what is or isn’t human, then we are no longer engaging in scientific research to distinguish what is human from what is not. Instead, we would merely be creating apologetic arguments to reinforce our preexisting biases.
It is certainly possible that a future statistical baraminology study could show evidence of continuity between Homo sapiens and creatures that are definitely not human, but such a study would have to be weighed in a holistic fashion against other evidences already uncovered and considered in hominin baraminology, including the evidence from Genesis 1 and 2. No single study should be considered absolutely definitive, especially if a study strongly contradicts previous research. More importantly, given the previous results of statistical baraminology studies of hominins, it seems more likely that future studies will continue to confirm and refine the continuity that unites all humans and the discontinuity that separates them from nonhumans. We can therefore look forward with anticipation to future discoveries to enhance our understanding of humanity and to improve our conceptions of discontinuity.
In that light, we can test the previous baraminology results of Wood (2016a) and O’Micks (2016a) that support including Homo naledi in the human holobaramin using the recently published character matrix of Dembo et al. (2016). Dembo et al.’s (2016) matrix consists of 391 craniodental characters and 24 taxa. For baraminic distance calculations, only taxa with taxic relevance of 0.3 or higher were evaluated. Baraminic distance correlation (BDC) and 3D multidimensional scaling (MDS) were calculated using BDISTMDS (http://www.coresci.org/bdist.html). After filtering for character relevance of 0.75, 140 characters were used to calculate baraminic distances. For BDC, 100 bootstrap pseudoreplicates were used to assess the sensitivity of BDC results to particular combinations of characters.
BDC results reveal one large cluster consisting of Homo species and Australopithecus sediba (fig. 1). Within the Homo cluster, all taxa share significant, positive BDC. Other clusters include Paranthropus and one cluster of chimpanzee and gorilla. No clusters share significant, positive BDC with any other clusters, and the only instances of significant, negative BDC occur between clusters. Most importantly for the matter at hand, Homo naledi shares significant, positive BDC with every other member of the Homo cluster, and bootstrap values for only two of these correlations are <90%. Homo naledi and Homo sapiens share significant, positive BDC with a bootstrap value of 100%.
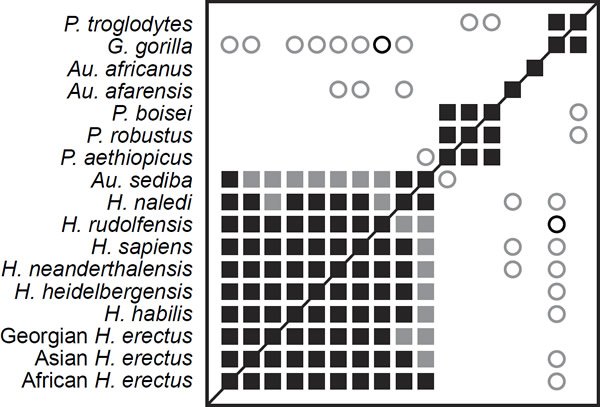
Fig. 1. Baraminic distance correlation results for the supermatrix of Dembo et al. (2016). Significant, positive BDC is indicated as a closed square; significant, negative BDC is indicated as an open circle. Black symbols indicate bootstrap values ≥90%; grey symbols indicate bootstrap values <90%.
The MDS results reflect the clusters observed in the BDC results (fig. 2). A. sediba and H. naledi appearing on the edge of the well-defined Homo cluster. The three-dimensional projection is of moderate quality, with a stress value of 0.16 and a minimal stress of 0.056 at seven dimensions. In the previous baraminology analysis of H. naledi from Wood (2016a), the characters from Berger et al. (2015) produced a comparable MDS result with a stress of 0.14 at three dimensions and a minimal stress of 0.07 at five dimensions. In the present results, H. naledi is not adjacent to any outgroup taxa from Paranthropus (mean baraminic distance 0.452), extant apes (mean baraminic distance 0.525), or A. africanus (baraminic distance 0.487) or A. afarensis (baraminic distance 0.563). The mean baraminic distance between H. naledi and other members of the Homo cluster is only 0.255.
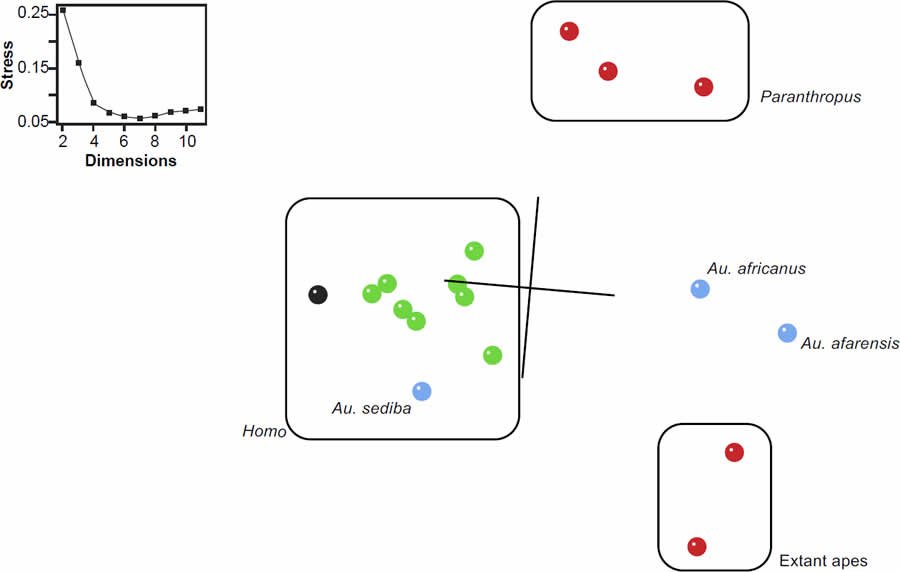
Fig. 2. Three-dimensional MDS results for the supermatrix of Dembo et al. (2016). Green symbols represent Homo species, red symbols are outgroups, and blue is Australopithecus. Homo naledi is shown in black. Stress values as a function of dimensions are shown in the inset.
Taken together then, these BDC and MDS results are consistent with the previous analysis of Wood (2016a). This confirmation should come as no surprise since Dembo et al.’s (2016) character matrix is a modified version of the same character data published in Berger et al. (2015), which formed the basis of Wood’s (2016a) original study. Instead, the present study is a refinement of Wood’s (2016a) study and is consistent with the conclusions of Wood (2016a), O’Micks (2016a), Wise (2016), and Line (2016) that Homo naledi is likely a member of the human holobaramin. Most importantly, the expanded sample of taxa and characters in Dembo et al. (2016) did not cause continuity to emerge between humans and nonhumans, as O’Micks (2016c) worried it might.
Conclusion
Even though the baraminology results and the evidence of intentional burial both support recognizing H. naledi as human, we still should not consider this question completely settled. O’Micks’ (2016b, c) concerns about the postcranial information are very important, and we should retain a small degree of skepticism regarding the validity of nonholistic baraminology studies. The future promises newly published details regarding the postcranial remains of H. naledi (e.g. Feuerriegel et al. 2016; Marchi et al. 2016), additional excavations in the Rising Star Cave, and possibly even Homo naledi DNA. In addition, the pending publication of detailed descriptions of StW 573, the Australopithecus “Little Foot” skeleton (see Clarke 2008), should provide a wealth of new information about presumably nonhuman hominins, against which the skeletons of putative humans like H. naledi and A. sediba can be compared. All of these new details will help us to revise our understanding of the human holobaramin, its variability, and its limits. Given our present understanding, though, we may tentatively accept Homo naledi as a human being descended from Adam and Eve and created in the image of God.
References
Berger, L. R., J. Hawks, D. J. de Ruiter, S. E. Churchill, P. Schmid, L. K. Delezene, T. L. Kivell, et al. 2015. “Homo naledi, a New Species of the Genus Homo from the Dinaledi Chamber, South Africa.” eLife 4: e09560.
Clarke, R. 2008. “Latest Information on Sterkfontein’s Australopithecus Skeleton and a New Look at Australopithecus.” South African Journal of Science 104 (11–12): 443–449.
De Miguel, C., and M. Henneberg. 2001. “Variation in Hominid Brain Size: How Much is Due to Method?” HOMO—Journal of Comparative Human Biology 52 (1): 3–58.
Dembo, M., D. Radovčić, H. M. Garvin, M. F. Laird, L.Schroeder, J. E. Scott, J. Brophy, et al. 2016. “The Evolutionary Relationships and Age of Homo naledi: An Assessment Using Dated Bayesian Phylogenetic Methods.” Journal of Human Evolution 97: 17–26.
Dirks, P. H. G. M., L. R. Berger, E. M. Roberts, J. D. Kramers, J. Hawks, P. S. Randolph-Quinney, M. Elliott, et al. 2015. “Geological and Taphonomic Context for the New Hominin Species Homo naledi from the Dinaledi Chamber, South Africa.” eLife 4: e09561.
Dirks, P. H. G. M., L. R. Berger, J. Hawks, P. S. Randolph- Quinney, L. R. Backwell, and E. M. Roberts. 2016. “Comment on: Deliberate Body Disposal by Hominins in the Dinaledi Chamber, Cradle of Humankind, South Africa?” Journal of Human Evolution 96: 149–153.
Falk, D., C. Hildebolt, K. Smith, M. J. Morwood, T. Sutikna, P. Brown, Jatmiko, E. W. Saptomo, B. Brunsden, and F. Prior. 2005. “The Brain of LB1, Homo floresiensis.” Science 308 (5719): 242–245.
Feuerriegel, E. M., D. J. Green, C. S. Walker, P. Schmid, J. Hawks, L. R. Berger, and S. E. Churchill. 2016. “The Upper Limb of Homo naledi.” Journal of Human Evolution. DOI:10.1016/j.jhevol.2016.09.013.
Gowlett, J. A. J. 2016. “The Discovery of Fire by Humans: A Long and Convoluted Process.” Philosophical Transactions of the Royal Society B 371 (1696): 20150164.
Green, R. E., J. Krause, A. W. Briggs, T. Maricic, U. Stenzel, M. Kircher, N. Patterson, et al. 2010. “A Draft Sequence of the Neandertal Genome.” Science 328 (5979): 710–722.
Harcourt-Smith, W. E. H., Z. Throckmorton, K. A. Congdon, B. Zipfel, A. S. Deane, M. S. M. Drapeau, S. E. Churchill, L. R. Berger, and J. M. DeSilva. 2015. “The Foot of Homo naledi.” Nature Communications 6: 8432.
James, S. R., R. W. Dennell, A. S. Gilbert, H. T. Lewis, J. A. J. Gowlett, T. F. Lynch, W. C. McGrew, C. R. Peters, G. G. Pope, A. B. Stahl, and S. R. James. 1989. “Hominid Use of Fire in the Lower and Middle Pleistocene: A Review of the Evidence [and Comments and Replies].” Current Anthropology 30 (1): 1–26.
Joordens, J. C. A., F. d’Errico, F. P. Wesselingh, S. Munro, J. de Vos, J. Wallinga, C. Ankjærgaard, et al. 2014. “Homo erectus at Trinil on Java Used Shells for Tool Production and Engraving.” Nature 518 (7538): 228–231.
Kivell, T. L., A. S. Deane, M. W. Tocheri, C. M. Orr, P. Schmid, J. Hawks, L. R. Berger, and S. E. Churchill. 2015. “The Hand of Homo naledi.” Nature Communications 6: 8431.
Laird, M. F., L. Schroeder, H. M. Garvin, J. E. Scott, M. Dembo, D. Radovčić, C. M. Musiba, et al. 2016. “The Skull of Homo naledi.” Journal of Human Evolution. DOI:10.1016/j.jhevol.2016.09.009.
Line, P. 2006. “The Mysterious Hobbit.” Journal of Creation 20 (3): 17–24.
Line, P. 2016. “The Mysterious Rising Star Fossils.” Journal of Creation 30 (3): 88–96.
Marchi, D., C. S. Walker, P. Wei, T. W. Holliday, S. E. Churchill, L. R. Berger, and J. M. DeSilva. 2016. “The Thigh and Leg of Homo naledi.” Journal of Human Evolution. DOI:10.1016/j.jhevol.2016.09.005.
O’Micks, J. 2016a. “Preliminary Baraminological Analysis of Homo naledi and its Place Within the Human Baramin.” Journal of Creation Theology and Science Series B: Life Sciences 6: 31–39.
O’Micks, J. 2016b. “Homo naledi Probably Not Part of the Human Holobaramin Based on Baraminic Re-Analysis Including Postcranial Evidence.” Answers Research Journal 9: 263–272.
O’Micks, J. 2016c. “Reply to ‘Taxon Sample in Hominin Baraminology: A Response to O’Micks.’” Answers Research Journal 9: 373–375.
Radovčić, D., A. O. Sršen, J. Radovčić, and D. W. Frayer. 2015. “Evidence for Neandertal Jewelry: Modified White-Tailed Eagle claws at Krapina.” PLOS ONE 10 (3): e0119802.
Randolph-Quinney, P. S., L. R. Backwell, L. R. Berger, J. Hawks, E. M. Roberts, G. Nhauro, and J. Kramers. 2016. “Response to Thackeray (2016)—The Possibility of Lichen Growth on Bones of Homo naledi: Were They Exposed to Light?” South African Journal of Science 112: a0177.
Rendu, W., C. Beauval, I. Crevecoeur, P. Bayle, A. Balzeau, T. Bismuth, L. Bourguignon, et al. 2014. “Evidence Supporting an Intentional Neandertal Burial at La Chapelle-aux-Saints.” Proceedings of the National Academy of Sciences USA 111 (1): 81–86.
Schoenemann, P. T. 2013. “Hominid Brain Evolution.” In A Companion to Paleoanthropology. Edited by D. R. Begun, 136–164. Chichester, West Sussex: Blackwell Publishing.
Schwartz, J. H. 2015. “Why the Homo naledi Discovery May Not be Quite What it Seems.” Newsweek, October 9, 2015. http://europe.newsweek.com/why-homo-naledi-discovery-may-not-be-quite-what-it-seems-332804.
Soressi, M., S. P. McPherron, M. Lenoir, T. Dogandžić, P. Goldberg, Z. Jacobs, Y. Maigrot, et al. 2013. “Neandertals Made the First Specialized Bone Tools in Europe.” Proceedings of the National Academy of Sciences USA 110 (35): 14186–14190.
Tyler, D. J. 2005. “The Amazing Shrinking Humans from Flores.” Origins 41: 8–11.
Welker, F., M. Hajdinjak, S. Talamo, K. Jaouen, M. Dannemann, F. David, M. Julien, et al. 2016. “Palaeoproteomic Evidence Identifies Archaic Hominins Associated with the Châtelperronian at the Grotte du Renne.” Proceedings of the National Academy of Sciences USA 113 (40): 11162–11167.
Wise, K. P. 2005. “The Flores Skeleton and Human Baraminology.” Occasional Papers of the BSG 6: 1–13.
Wise, K. P. 2016. “Paleontological Note on Homo naledi.” Journal of Creation Theology and Science Series B: Life Sciences 6: 9–13.
Wood, B., and E. K. Boyle. 2016. “Hominin Taxic Diversity: Fact or Fantasy?” Yearbook of Physical Anthropology 159: S37–S78.
Wood, T. C., K. P. Wise, R. Sanders, and N. Doran. 2003. “A Refined Baramin Concept.” Occasional Papers of the BSG 3: 1–14.
Wood, T. C. 2010. “Baraminological Analysis Places Homo habilis, Homo rudolfensis, and Australopithecus sediba in the Human Holobaramin.” Answers Research Journal 3: 71–90.
Wood, T. C. 2011. “Baraminology, the Image of God, and Australopithecus sediba.” Journal of Creation Theology and Science Series B: Life Sciences 1: 6–14.
Wood, T. C. 2016a. “An Evaluation of Homo naledi and ‘Early’ Homo From a Young-Age Creationist Perspective.” Journal of Creation Theology and Science Series B: Life Sciences 6: 14–30.
Wood, T. C. 2016b. “Taxon Sample Size in Hominin Baraminology: A Response to O’Micks.” Answers Research Journal 9: 369–372.
Zilhão, J., D. E. Angelucci, E. Badal-García, F. d’Errico, F. Daniel, L. Dayet, K. Douka, et al. 2010. “Symbolic Use of Marine Shells and Marine Pigments by Siberian Neandertals.” Proceedings of the National Academy of Sciences USA 107 (3): 1023–1028.








