Serratia marcescens, the “Flame” Strain: The Genesis of a New Variant
A Newly Described Strain with Prolific Pigment Produced at High Temperature
Abstract
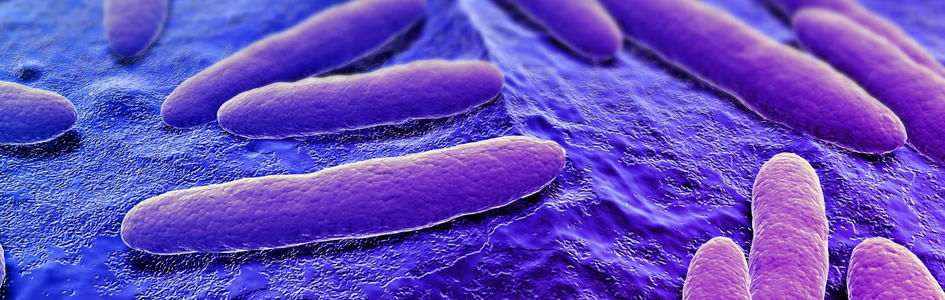
Serratia marcescens, a Gram-negative, rod-shaped, facultative anaerobe (Fig. 1), is ubiquitous in water, soil, and natural settings. It is easily grown in the lab and may serve as an ideal model for adaptation studies because of the natural color variation of S. marcescens (Gillen 2008). In this paper, we describe a new variant with prolific pigment (prodigiosin) production at high temperatures. In the wild and in buildings, S. marcescens is noted for the production of a bright red pigment called prodigiosin (Williams 1973). We have found a new strain that appears to have adapted to a relatively new pond system called Liberty Library Lake. It produces pigment up to 40°C without any enrichment to media. Most wild-type strains, like NIMA, produce pigment normally up to 30°C, but with extensive enrichment, wild-type strains can produce pigment up to 40°C. This new strain, called the “Flame” strain, not only produces prodigiosin to 39–40°C but also in higher abundance at 35°C and at a brighter hue. NIMA strains can produce pigment at 39–40°C with Serratia Synergy Agar (glycerol, peptone, agar) but not on TSA nor any common agar. It takes significant enhancement for any other Serratia marcescens strains to produce pigment even at 35°C.
The Flame strain’s brief appearance in a local, small lake appears to be a phenotypic diversification and adaptation to an environmental perturbation this past school year. The environmental stress prior to its appearance was an autumn drought. Eventually, heavy rainfall occurred and the new strain was discovered. Its appearance coincided with an unusually high abundance of coliforms, avian Giardia, and Cryptosporidium, along with chemical treatment of the lake. The unusual conditions seem to favor a rapid phenotypic diversification and adaptation. The new strain still retains the pigment production at nearly 10°C higher for “normal” prodigiosin production by wild-type Serratia marcescens. This genesis of this new strain seems to have occurred as special conditions favored this new variant. It may be closer to a “proto-type” (ancestral) strain than to more common wild-type strains, like NIMA and BS303. It appears that most wild-type strains, like NIMA and BS303, may have lost this information over time since added enrichment is necessary to produce pigment at 39–40°C. The unusual conditions may have selected for this newly adapted strain to be common for a short time. Also as conditions returned to “normal,” a common wild-type strain reappeared at the local lake, and the Flame strain was no longer found.
The objective of this article is to explain the mysterious origin of a new strain of Serratia marcescens that produces prodigiosin up to 40°C without any enrichment to media. This strain can naturally produce prolific pigment that is a bright, flame-red. Since Serratia marcescens offers protection from other microbes, UV light, and drought, it is a wonderful example of intelligent design commonly seen in the microbial world.
Introduction
Serratia marcescens is best known for its red or pink pigment (Fig. 1). The most salient, signature feature of Serratia marcescens is its ability to produce this beautiful red pigment (prodigiosin) at room temperature. Most strains produce abundant pigment from 25–30° C, although the bacteria grow from 4–42°C; the pigment may form at 12–36°C in some strains (Williams and Quadri, 1980). Prodigiosin comes from the Latin word prodigiosus, which means wonderful, amazing, miraculous, unnatural (supernatural), divine, or dealing with wonders. In 1848, Ehrenberg named the organism Monas prodigiosus, or the “miracle bacterium,” which was later modified to Bacillus prodigiosus. By the 1920s, revisions in taxonomy of bacteria as well as the desire to recognize the work of Bizio led to the gradual adoption of the name originally proposed by Bizio (i.e. Serratia marcescens), although we still refer to it as the “miracle” bacilluS. marcescens means decay, and it may refer to the fact that the pigment will often disappear in aged colonies without enriched media. The name derives from the Latin marcescere, meaning to wither or decay, because of the properties of the organism to lose its reddish color.
Serratia Variants and the Flame Strain
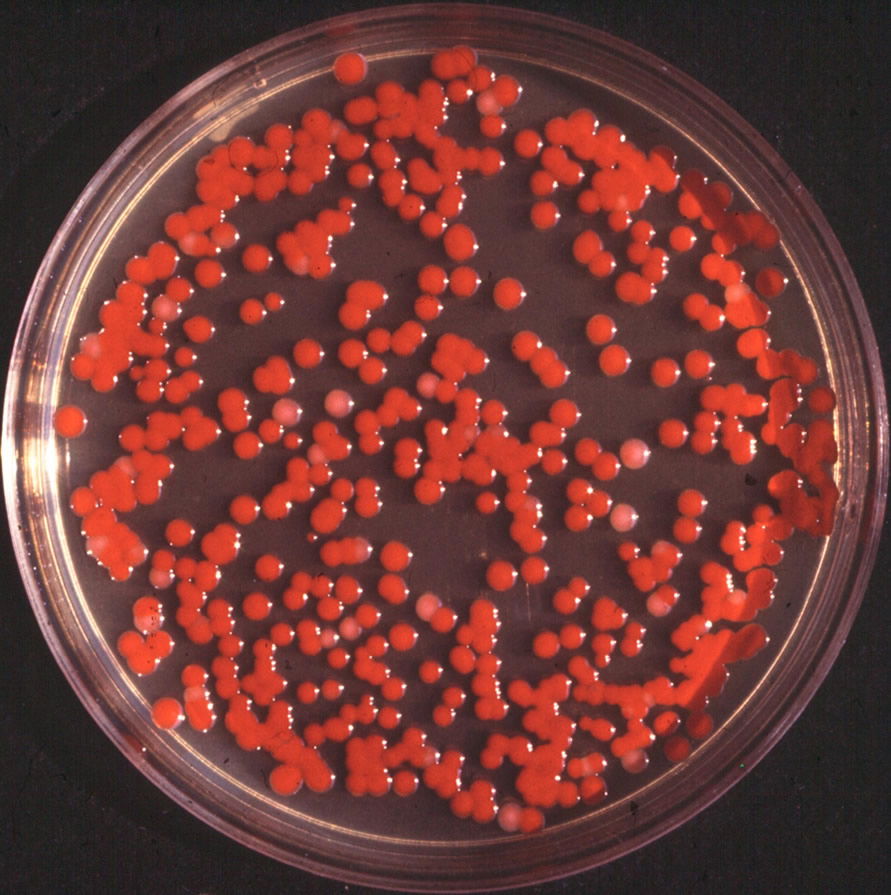
Figure 1. Serratia marcescens typical colony. By de:Benutzer:Brudersohn, via Wikimedia Commons.
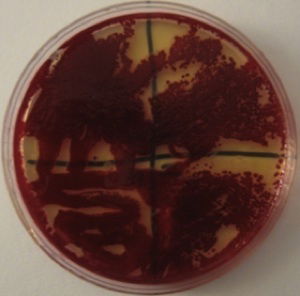
Figure 2. Serratia marcescens blood-red colonies. Image by Alan L. Gillen.
Although most microbiologists know that S. marcescens produces the bright red pigment prodigiosin, not many know that some Serratia mutants can be white, purple, pink, orange, and even blue. It produces a wide diversity of color morphs, depending on the partial or complete synthesis of prodigiosin (Williams 1956, 1973) and other pigments. Serratia can also turn a deep blood-red color (Fig. 2), but for this to occur the bacteria “culture” needs to age (be older than 8 days) and the substrate needs to have a form of glycerol (oil) and is low in phosphate and sugar. It has long been known that preparing the culture with low phosphate and low glucose solutions can enhance the intensity of the pigment in broth or agar, allowing Serratia to produce enough prodigiosin to have a purple, blood-red, crimson, or maroon appearance, not just bright red or pink. It can also produce other color pigments beyond prodigiosin.
We have been particularly interested in what controlled the expression of the red phenotype in S. marcescens for many years. Pigment production in Serratia is influenced by several variables, including temperature, nutrient media, and exposure to ultraviolet (UV) light (Gillen and Gibbs 2011). Prodigiosin ranges in color from dark red to pale pink, depending on the temperature, substrate, and age of the colonies. Most strains of S. marcescens are red under 27ºC and white above 28ºC. (NIMA strain pigment and flagella production frequently slows at 28º C.) NIMA wild-type is characterized by its most common colors: pink and red. Our new strain produces a bright flame-red pigment up to 40ºC; which is significantly higher than most and 3°C higher than any reports. It is a very prolific prodigiosin pigment producer at 25–35°C. This new strain produces the pigment faster than NIMA (typical wild-type) and also with great intensity and without extra enriched media ingredients (such as glycerol and peptone). We named it “Flame” strain both because it appears flame red and because the athletic teams at Liberty University are named the “Flames.” The red pigment offers protection against UV in sunlight, serves as an antibiotic, and has cytotoxic qualities.
Field and Lab Work, and Isolation
The strain was first noticed in mid-October 2017 but was not clearly identified until October 31 by Gram stain, Enterotube II (Fig. 3), and other biochemical tests. The strain was never found in duck or geese feces. Only Cryptosporidium, Giardia, Klebsiella, and Citrobacter were found in avian feces. The most likely place of the Flame strain’s origin was mountain soil and runoff into the lake (Fig. 4). Chandler Mountain overlooks Liberty University’s Library Lake.
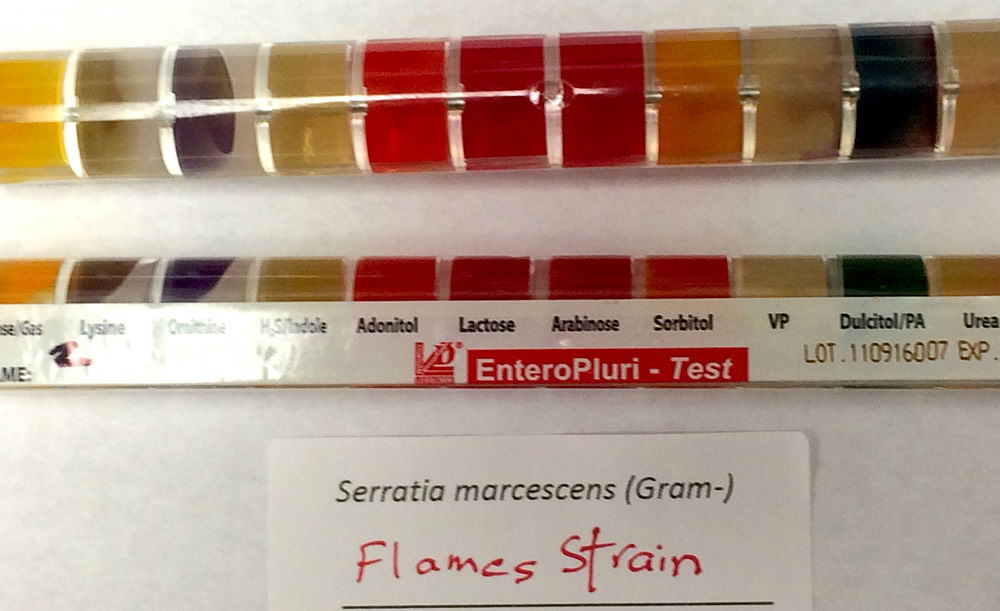
Figure 3. Enterotube Results. Image by Alan L. Gillen.

Figure 4. Library Lake Aerial view. Arrow indicates the location where the Flame strain was isolated. Modified Google image.
We conducted a series of experiments during the spring 2018 semester as a follow-up to the Flames strain’s isolation in the fall. The main series of experiments focused on determining the range of temperatures at which prodigiosin would be produced. Samples of the NIMA and Flame strains were incubated on Trypticase Soy Agar (TSA) at temperatures ranging from 25–40°C at 24 and 48 hours intervals. After the 24 and 48 hours had elapsed, the plates were retrieved from the incubators, photographed, and analyzed. This process was repeated on a special kind of medium called Serratia Synergy Agar (SSA.) SSA was prepared by combining Bacto Peptone, agar, and glycerol in water. Once the 24 and 48 hours had elapsed, the plates were removed from the incubators, photographed, and analyzed.
In addition to growing the various strains on agar, attention was directed at determining any chemical differences between the two samples of prodigiosin produced at different temperatures. This was done in an attempt to explain why there was a difference in the temperatures at which they were produced. The pigments were analyzed through high-performance liquid chromatography (HPLC) with the assistance of biochemistry professor Dr. Greg Raner at Liberty University. The following procedure was performed in duplicate, once for the NIMA strain and once for the Flame strain. Prodigiosin was extracted from the Serratia marcescens cells, then removed and placed in HPLC tubes. The analysis medium was a 70:30 ratio of methanol to deionized water. The resulting chromatograph was photographed (Fig. 9).
Results and Discussion
The results of the research and experimentation showing high levels of pigment production for such high temperatures were surprising. Therefore, a wide variety of tests were performed to analyze the differences between the NIMA strain and the Flame strain of Serratia marcescens. Serratia marcescens strains may be seasonal and grow better in the autumn than in the spring. The Flame strain was found under the following conditions of the lake: clear, cool, autumn-like conditions, after leaf fall, with an abundance of coliforms (Klebsiella, Citrobacter, E. coli) and the presence of Giardia and Cryptosporidium. Another factor that may have influenced its presence was the treatment of the lake with blue dye to make the lake look more attractive. Treatment with these chemicals might have influenced the phenotypic expression and adaptation of Serratia marcescens present in the lake.
After bacteria isolation, we examined the differences in the NIMA strain and the Flame strain (Figs. 5, 6, 7, and 8). Two different tests were performed to ascertain any differences between the two strains. The first series of tests involved growing the bacteria on two different kinds of agar at varying temperatures. The second test (Fig. 9) involved analyzing the prodigiosin produced by each strain through high performance liquid chromatography (HPLC) to see if there were any chemical differences between the two pigments produced. The results of the prodigiosin production for varying temperatures between the two strains showed the NIMA strain clearly produced prodigiosin at 28°C and produced a much lower amount of prodigiosin at 30°C on TSA. However, the Flame strain clearly produced abundant prodigiosin from 28–35°C. The production of prodigiosin in the Flame strain was significantly reduced at 37°C but continued for a few colonies up to 40°C. In summary, the NIMA strain stopped producing prodigiosin at 30°C and the Flame strain stopped producing prodigiosin at 37°C. Therefore, there is a clear distinction between these two strains.
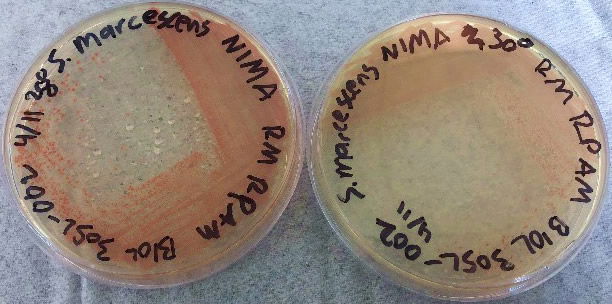
Figure 5. The NIMA strain of S. marcescens on TSA at 28–30°C. Image by Ryan MacKay.
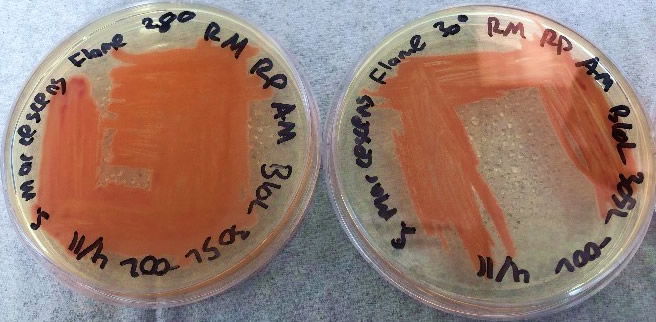
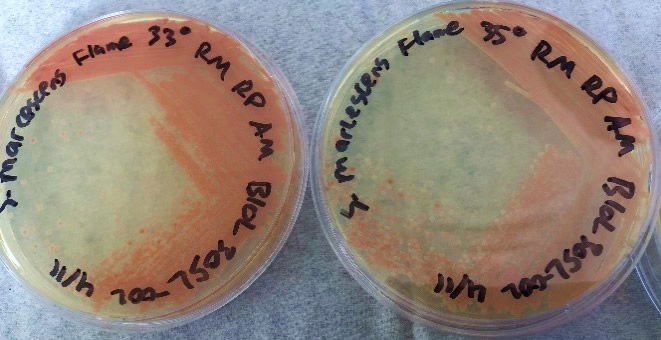
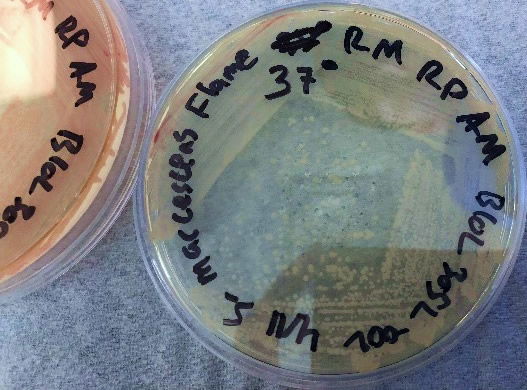
Figure 6. The Flame strain of S. marcescens on TSA at 28–37°C. Image by Ryan MacKay.
The results using the Serratia Synergy Agar (SSA) plates (Fig. 7) were similar to the previous tests (Fig. 6), with the pigment from the Flame strain appearing at higher temperatures than that of the NIMA strain. However, using the SSA plates we were able to see the NIMA strain produce prodigiosin at a higher temperature than before, lasting now until 37°C. Also, the Flame strain produced prodigiosin on the SSA plates up to 40°C. Although the NIMA strain was producing prodigiosin at higher temperatures on the SSA plates than the TSA plates, it was still not producing prodigiosin at the same temperatures as the Flame strain.
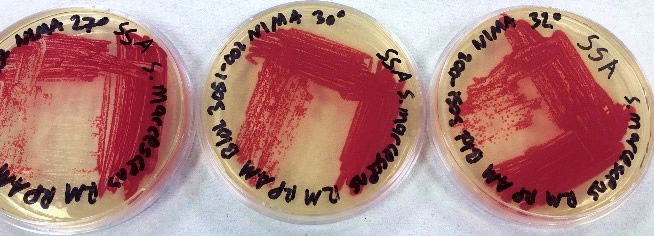
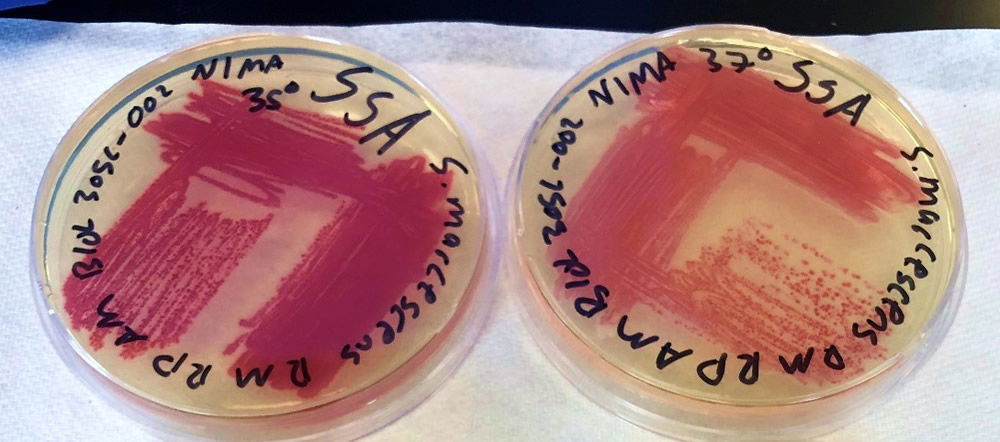
Figure 7. The NIMA strain of S. marcescens on SSA at 27–40°C. Image by Ryan MacKay.
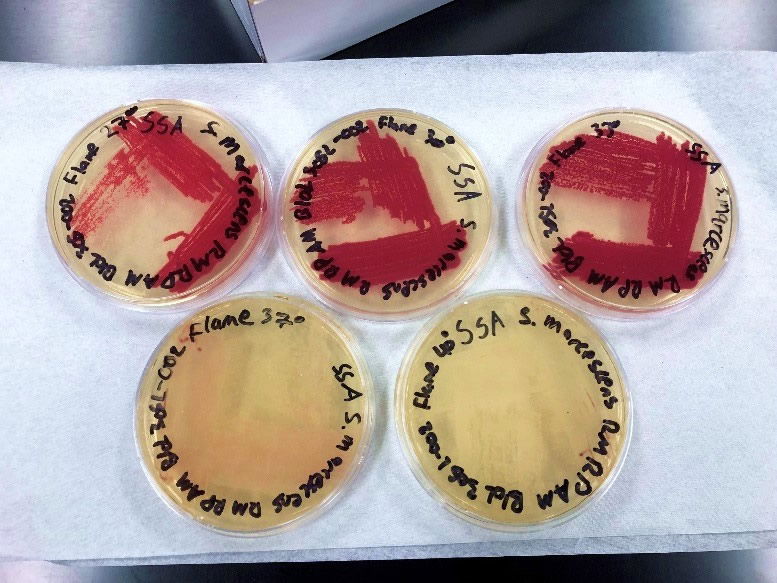
Figure 8. The Flame strain of S. marcescens on SSA at 27–40°C. Image by Ryan MacKay.
The pigments produced by the two strains were analyzed to ascertain if there was a chemical difference between the two strains (Fig. 9). The high-performance liquid chromatography (HPLC) indicated that the pigment of the two strains was chemically identical and that there was no chemical reason for the higher temperature production of prodigiosin in the Flame strain. This is another example of how the Flame strain is different from the NIMA strain of Serratia marcescens.
As displayed in the chromatograph, the retention times for the Flame strain and NIMA strain, 2.308 and 2.299 minutes, were almost identical; this indicates the same prodigiosin was produced in both strains. The variance in absorbance displayed by the height of the peaks does not indicate a difference in chemical makeup, but rather a stronger concentration of prodigiosin in the Flame strain. Therefore, the production of prodigiosin in the Flame strain at higher temperatures is not related to a chemical difference of the prodigiosin itself.
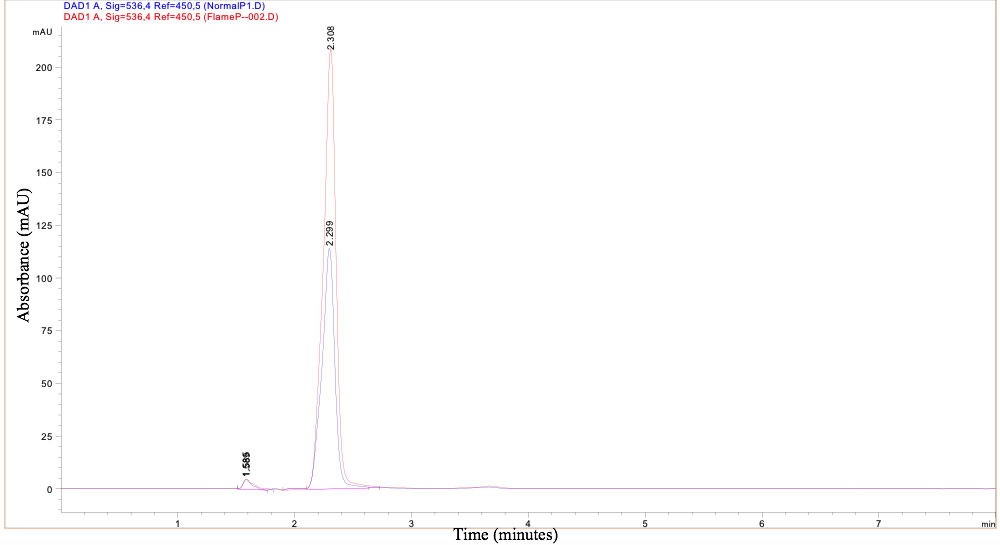
Figure 9. HPLC Chromatograph of prodigiosin produced by the Flame and NIMA strains of S. marcescens. Ryan MacKay image
Prodigiosin is a time-delayed, secondary metabolite constructed from several amino acids that may accumulate in the cell as a result of primary metabolism (Williams 1973). (Antibiotics produced by bacteria are also secondary metabolites. They are not essential for survival, but are very helpful in establishing bacteria’s domain in soil or water.) Four amino acids are needed: proline, methionine, histidine, and alanine. In the lab, they are often derived from peptone and glycerol. Proline is incorporated intact in the prodigiosin molecule; histidine is used indirectly; methionine contributes a methyl group; alanine is entirely incorporated except for a carboxyl group. Prodigiosin, a linear tripyrrole, is synthesized in a bifurcated pathway, in which precursors are synthesized separately and then couple to form the red pigment (Fig. 5). There at least 10 different enzymes involved in its synthesis (Williams 1973). The synthesis of prodigiosin is irreducibly complex.
Quorum Sensing and 15 Genes Control Pigment Production
Quorum sensing is a means by which bacterial cells communicate. The relationship between cell density and pigment production has led some scientists to propose that this pigment is a secondary metabolite associated with quorum sensing gene expression patterns. Each cell releases signaling molecules that other nearby cells of the same species can detect. As the density of the population increases, so does the abundance of the signaling molecules. These signaling molecules in turn regulate gene expression in such a way that when a certain abundance of signaling molecules (and thus cells) exists, the cells alter their gene expression to change their metabolic strategy accordingly. This allows bacterial populations to maximize their efficiency in dealing with a rapidly changing environment, potentially even cooperatively coordinating their metabolic processes.
In quorum sensing, genes are only expressed when the density of the bacterial population reaches a critical point of need. This regulated expression is now recognized as an important communication and swarming means used by a variety of different bacteria. In recent years, we have found that quorum sensing and genes control this prodigiosin expression at a molecular level. In this model, as with Serratia, population size increases, and their gene expression pattern changes accordingly. Consequentially, some new metabolic pathways are initiated, a byproduct of which is the prodigiosin pigment.
This cell–cell communication relies on the principle that when a single bacterium produces low-molecular-mass–signaling molecules, the extracellular concentration is below a certain threshold. The production of prodigiosin in Serratia is dependent on the pig cluster, comprising 15 genes Briefly, MAP (2-methyl-3-amylpyrrole) and MBC (4-methoxy-2,2′-bipyrrole-5-carboxyaldehyde), formed from the precursors acetate, serine, alanine, methionine, and proline, are believed to be joined in a condensation reaction to prodigiosin (Williamson et al., 2005). NIMA, WF, and 933 were first figured out by R.P. Williams and students in the 1970–80s.
As previously pointed out (Gillen and Gibbs 2011), the synthesis of the pigment is an intricate, complex process to form prodigiosin. The pig genes for prodigiosin and preadaptation to a new environment allows for the rapid change in that a complex of DNA (PigP homolog) controls prodigiosin biosynthesis in a wide range of temperatures and substrates available. When more pigment is produced, it protects the bacteria (an adaptation). The reason for this gene coding is likely to be pre-existing DNA coding that regulates according to need. Adaptive mutations as described by Barry Hall can readily fit within a preadaptive mechanism and are a design feature of bacteria. Many biologists have a full appreciation of the plasticity of genes for pigments and the capacity of bacteria to rapidly adapt to new environments. This helps to explain a very good design for variation in nature.
The Genesis of a New Variant or the Appearance of an Ancestral Prototype?
Does the Flame strain represent a new variant or the reappearance of an ancestral prototype? It probably displays properties of a new variant, but it seems to retain more of the ancestral prototype information than most living strains today. We report here the characterization of this variant to have not only a greater abundance of prodigiosin at higher temperatures than the NIMA wild type, but to also be more resistant to UV light. The morphological variants differed extensively from the NIMA wild type, BS 303wild type, and WF, 933 in UV light experiments; and they have great longevity in the lab.
Heat, Drought, Leaf Enrichment, Biofilms, and LU Lake “Environment”
LU Lake is an artificial system of four connected ponds created around 2012 to help with the drainage system around the Vines Center. It is relatively new and constantly changing. The Flame strain’s brief appearance in LU Library Lake appears to be a phenotypic diversification and adaptation to an environmental perturbation this past school year. Its appearance coincided with an unusually high abundance of coliforms, avian Giardia and Cryptosporidium, along with chemical treatment of the lake. In addition, this was the peak of the hot, dry autumn season, and the nearby mountain would have indirectly sent tree leaves and other organic matter into the water drainage system. This, in turn, changed the microbiota and the biofilm formation in the lake. The unusual conditions seem to favor a rapid phenotypic diversification, and this adapted strain still retains the pigment production at nearly 10°C higher than normal prodigiosin production by Serratia marcescens. This strain appears to be closer to a proto-type (ancestral) strain than to wild-type strains like NIMA and BS303. Most wild-type strains, like NIMA and BS303, seem to have lost post-translational regulatory information over time. The unusual conditions may have selected for this newly adapted strain to be common for a short time. As conditions returned to normal, the wild-type strain has re-established its dominance in the local lake.
Adaptive mutations can readily fit within a creation model where adaptive mechanisms are a designed feature of bacteria. Further understanding of these mutations in the PigP homolog may help the development of a creation model for adaptation of bacterial populations in response to the changing environmental conditions, for example, in a pond or lake. In our case, the drought for six weeks prior to the Flame strain’s appearance was the adversity.
Dr. Georgia Purdom (2013) summarizes the work of Barry Hall on adaptive mutations in explaining beneficial mutations from a creation model. Decades of research by Dr. Barry Hall indicates adverse condition, like starvation or drought, may initiate mechanisms in bacteria that result in mutation that specifically allows them to survive, grow, and thrive in the altered environment. These changes do not appear to be random, in respect to the environment, thus the term directed, or adaptive, mutations is used.
Adaptive mutations are problems for Darwinian evolutionists because organisms are responding to specific environments by adaption with pre-existing traits and set limits (boundaries) on the genetic change. The mechanisms cannot account for the origin of novel traits. This seems to be common in E. coli and other bacteria. Only a rapid change in salt content has been studied in Serratia marcescens “evolution.” A gene cannot just become completely different; adaptive mutations are limited. Mutations can cause changes in pre-existing traits, and selection pressures can act upon them; however, they cannot account for totally novel traits necessary for molecule-to-man evolution.
S. marcescens showed its ability for adaption when subjected to a highly salty environment (80g/L or 100g/L); the bacteria treated in the salty conditions displayed an elevated level of salt tolerance that was not found in the samples exposed to a less salty environment (Ketola and Hiltunen 2014). Similarly, the Flame strain appears to have acquired a strong level of prodigiosin production at high temperatures in response to changing environmental conditions, probably drought.
Microbial persistence in a stressful environment or in animal hosts has been described as survival in the “change” fast track. In the present study, we have demonstrated that within a highly controlled environment, microorganisms respond through phenotypic diversification to enhance the adaptive potential of the microbial population. Moreover, specialized traits displayed by different phenotypic variants correlated with specific stages of biofilm development in other studies of Serratia marcescens. Hot, drought-like conditions preceded the appearance of Serratia’s Flame strain. In addition, it appears that the seasonal leaf addition during autumn was a contributing factor in biodiversity of bacteria in the lake and biofilm. Through the month of September and early October, the diversity of bacteria increased, especially the coliform population. This appears to be the key to the adaptive mutation that Barry Hall describes. This has been called mediated design by creationists. God designed created kinds with genes that could be turned on to help adapt to new environments, similarly to campers who take Swiss army knives with them to face unknown challenges in the wilderness. The diversity of tools serves different functions for specialized needs in camping, fishing, and survival (Purdom 2013).
Some adaptive design traits can only be expressed under certain conditions to allow microbes, plants, animals, and humans to fill the earth as environments change over time (Genesis 1 and 8). God programmed organisms with mechanisms that would be triggered under certain conditions to allow organisms to survive, thrive, and to function in new environments (Purdom 2013). In the case of Serratia marcescens, prodigiosin provides protection and enhances survival.
Prodigiosin Pigment Offers Protection and Enhances Survival
The ability to produce prodigiosin over a wider temperature range would have afforded Serratia extra protection during the six-week drought that occurred in September/October 2017. The functions of pigment have long been pondered but only recently determined (Gillen and Gibbs 2011). It appears that prodigiosin offers protection for Serratia in the natural environment. It appears that it is worth the energy investment to synthesize prodigiosin when it serves as protection against UV light and when it has to compete with fungi in the soil, using this red pigment as an antibiotic against neighboring molds and bacteria. During drought, the Flame strain provides something more than most wild-type strains.
Prodigiosin is a purposeful pigment that is beautiful and enhances survival of Serratia marcescens in nature. Serratia marcescens is a beneficial microbe in that it recycles elements in nature, like breaking down fallen leaves in the autumn. In addition, it produces a natural antibiotic (part of normal microbiota) protecting wildlife, like frogs. Prodigiosin has even been used as an anticancer agent (Coley’s toxin) by William B. Coley and thousands of other physicians since the early 1900s (Tortora, Funke, and Case 2018); it is still used today in Germany.
The Creator’s Signature
The pigment does in fact have a brilliant red color that attracts attention, and its natural production of variable bright colors testifies of the Creator’s artistic abilities. When viewed in the Petri dish, or up close, it is a highly attractive microbe. Finally, ability of the bacterium to produce the pigment and adapt under varying environmental conditions suggests the Sustainer’s foreknowledge of S. marcescens need to survive. Sometimes beautiful things come out of drought or storms—beauty from ashes (Isaiah 61:3). In this case, a brighter, longer-lasting red signature bacteria. “Behold, I am doing a new thing; now it springs forth, do you not perceive it?” (Isaiah 43:18).
We should not be surprised that bright pigments in nature remind us that beauty is a part of God’s plan. In Matthew 6:28–29, Jesus reminds us that God arrays lilies with color and beauty, providing soil and sun. He consistently cares and provides for his creation. If we extend this principle to bacteria and the pigment of S. marcescens, God provides prodigiosin for protection, survival, and extension of its domain in soil and water.
References
Gillen, A. L. 2007. The Genesis of Germs: Disease and the Coming Plagues in a Fallen World. Green Forest, AR: Master Books.
Gillen, A. L. and R. Gibbs. 2011. Serratia marcescens: The Miracle Bacillus. Answers in Depth 6 (July 20, 2011): https://answersingenesis.org/biology/microbiology/serratia-marcescens-the-miracle-bacillus/.
Gillen, A. L. and F. Sherwin. 2017. The Design of Giardia and the Genesis of Giardiasis. Answers in Depth 12 (July 19, 2017): https://answersingenesis.org/biology/microbiology/design-giardia-and-genesis-giardiasis/.
Ketola, T. and T. Hiltunen. 2014. Rapid Evolutionary Adaptation to Elevated Salt Concentrations in Pathogenic Freshwater Bacteria Serratia marcescens. Ecol Evol 4, no. 20 (September 23, 2014): 3901–3908, doi:10.1002/ece3.1253.
Purdom, G. 2013. What About Beneficial Mutations? Chapter 24 in The New Answers Book 4. Green Forest, AR: Master Books.
Tortora, G.J., B. R. Funke, and C. L. Case. 2018. Microbiology, An Introduction. 13th ed. San Francisco, CA: Pearson Benjamin/Cummings Pub. Co.
Williams, R. P. 1973. Biosynthesis of Prodigiosin, a Secondary Metabolite of Serratia marcescens. Appl. Microbiol. 25 (3): 396–402.
Williams, R. P., and S. M. H. Qadri. (1980). The Pigments of Serratia. A. Von Graevenitz and S. J. Rubin, (eds). The Genus Serratia. Boca Raton, FL: CRC Press, 31–75.
Williams, R. P., J. A. Green, D. A. Rappoport. 1956. Studies on Pigmentation of Serratia marcescens. I. Spectral and Paper Chromatographic Properties of Prodigiosin. J Bacteriol 71 (1): 115–120.
Williamson, N. R. et.al. 2005. Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2‐methyl‐3‐n‐amyl‐pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. 07 April 2005. Molecular microbiology. doi:10.1111/j.1365-2958.2005.04602.x.
Appendix
Serratia
Serratia bacteria will grow in any damp location (water) where phosphorous-containing materials or fatty substances reside. Some phosphorous has been reported in the pond system (LU Library Lake). The ducks and geese have a “greasy” stool to our observations. Pigment production can be artificially enhanced by glycerol, peptone, fatty substances, and phosphorous. Serratia Synergy Agar (SSA) contains glycerol and peptone in addition to conventional agar. We used SSA because it is similar to LU Library Lake conditions.
| Name and Description | ATCC® Number | Antibiotic Resistance |
|---|---|---|
| 1. Nima (D-3) (Wild-type, TMC, Blood Red) | 29632™ | Resistant |
| 2. 9-3-3 (Clinical Mutant TMC, white) | 29634™ | Resistant |
| 3. WF (Clinical Mutant, white-pink) | 29635™ | Resistant |
Possible Formal Name
A formal name proposed for this new strain is Serratia marcescens RPW—RPW for Robert P. Williams. This strain is dedicated to Dr. Robert P. Williams. Dr. Williams was the president of American Society for Microbiology (ASM) from 1983–1985. He was a humble, brilliant researcher on S. marcescens and many other bacteria, and spent over 43 years studying prodigiosin from 1949–1992: not a casual observer. He published 128 peer-reviewed papers, wrote 15 chapters for books, and authored his own microbiology textbook (Burdon, K. L., and R. P. Williams. 1968. Microbiology, 6th ed. New York: Macmillan Co.).
Acknowledgements
We wish to express our appreciation to Dr. Greg Raner for helping us with the chromatography work and also to Dr. Georgia Purdom for both pioneering the concept of adaptive mutations from a design point of view and her providing feedback on this manuscript.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- © 2024 Answers in Genesis


