
Iron Key to Preserving Dinosaur Soft Tissue
Abstract
Iron may paradoxically be the key, claim evolutionist researchers, to preserving dinosaur soft tissue for evolution’s assumed millions of years. More specifically, highly reactive iron atoms are released from proteins when an organism dies; while the organism is alive, iron is sequestered in useful proteins, thus preventing it from participating in destructive chemical reactions. It remains impossible to demonstrate just how long such preservation has lasted, despite the evolutionist claims that iron-induced preservation could last millions of years.
Plausibility of Preservation
The key to preserving dinosaur soft tissue may literally be made of iron. In Dr. Mary Schweitzer’s quest to go beyond demonstrating the authenticity of ancient dinosaur soft tissue to determining the mechanism for such preservation, the North Carolina State University paleontologist thinks iron is the answer. Iron atoms released at death from hemoglobin may, she believes, preserve biomaterials through deep time.
Debate has raged ever since 2005, when Mary Schweitzer identified soft tissue containing red blood cells and blood vessels inside dinosaur bone. How could soft tissue be preserved inside a dinosaur bone? Could protein and cellular structures survive for millions of years? At the October 2012 meeting of the Society of Vertebrate Paleontologists, Schweitzer presented clear evidence that proteins found only in vertebrates and even the basic structure of bone cells were preserved in the dinosaur bones she was studying. Now Schweitzer’s group has unmasked a molecular mechanism that may well account for preservation of tissues after death, and they have demonstrated its ability to preserve soft tissue for two years. Schweitzer believes that if the mechanism will keep tissue pliable and intact for two years, then why not millions.
Locking up Iron
Hemoglobin—the oxygen-carrying protein in red blood cells—is bound, or chelated, to iron atoms. Iron is normally highly reactive, and it is just that tendency of iron to react readily to oxygen that makes hemoglobin a great oxygen-carrying protein. Hemoglobin, by chelating iron, harnesses iron’s reactive nature for the purpose of carrying oxygen in blood.
Hemoglobin and other iron-containing proteins like ferritin1keep iron atoms safely sequestered where they can do their jobs without generating dangerous oxygen-free radicals. Free radicals damage biomolecules by cross-linking their parts—essentially freezing their overall structures while making them non-functional. But after an organism’s death, its protein molecules tend to denature—to unravel and become useless—or to be degraded by the actions of microbes or any remaining proteolytic (protein-destroying) enzymes. Denatured hemoglobin and ferritin can no longer hold onto their iron atoms.
Could Iron Nail Down Biomolecular Structure?
Once unleashed from chelating proteins like hemoglobin by death, chemically reactive iron atoms are free to react with other molecules. Ironically, Dr. Schweitzer notes that—by reacting with certain amino acids on nearby proteins—when iron destructively chelates and cross-links those molecules, it stabilizes their structure. This would ordinarily render them biologically inactive in a living organism and seems to make them harder to detect using modern methods, but such cross-links fix the molecules, protecting them from further destruction.
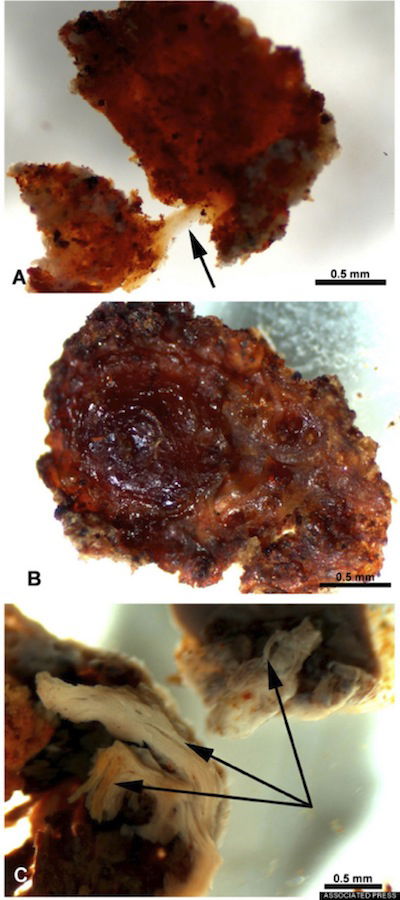
This fragment of demineralized tissue lined the marrow cavity of a T. rex femur. The demineralized fragment in “A” was flexible and resilient. When stretched, as shown by the arrow, it returned to its original shape. “B” shows the same fragment after air-drying. The fibrous character of the fragment is shown in “C.” Image from Associated Press/Science/Huffington Post.
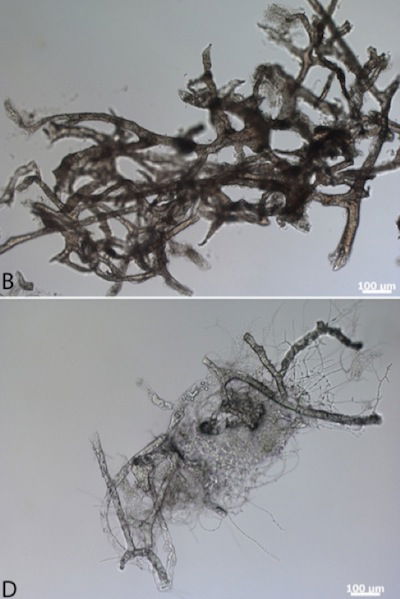
On the top are ostrich blood vessels that were soaked in a hemoglobin solution for five days to simulate exposure to lysed red blood cells soon after death. In the bottom photo are ostrich vessels soaked only in water. These photos show that after thirty days the hemoglobin-soaked vessels are well-preserved, whereas the control group are decaying. Electron microscopy confirmed the excellent preservation conferred by hemoglobin exposure. Schweitzer believes that the iron in the hemoglobin is the substance that prevents tissue degradation. Image by Mary H. Schweitzer et al., via Royal Society Publishing.2
This fixation process can happen not only to protein molecules but also to DNA, and last year Schweitzer did report the presence of what might be DNA fragments in dinosaur bone she was examining. Furthermore, any proteolytic enzymes near iron atoms could easily be deactivated by such cross-links between their amino acids, further forcing tissue degradation to grind to a halt.
“Iron is necessary for survival, but it’s also highly reactive and destructive in living tissues, which is why our bodies have proteins that transport iron molecules to where they are needed but protect us from unwanted reactions at the same time,” Schweitzer says. [A thoughtful person might ask how such a beautifully designed system for life came into existence by blind, purposeless, directionless evolutionary processes, but we digress.] She continues, “When we die, that protective mechanism breaks down and the iron is turned loose on our tissues – and that destructive process can act in much the same way formaldehyde does to preserve the tissues and proteins.”3 Schweitzer says, “The free radicals cause proteins and cell membranes to tie in knots. They basically act like formaldehyde.”
The Iron Key to Softness
Schweitzer first reported the presence of dinosaur soft tissue, which turned out to be collagen consistent with blood vessels along with red blood cells, in the thigh bone of a juvenile T. rex in Montana. “What we found was unusual, because it was still soft and still transparent and still flexible,” she says. Her 2005 discovery excited much controversy in the evolutionary community, as it seems quite impossible that anything could preserve something so chemically “fragile” for millions of years. Evolutionists date the first dinosaur in which Schweitzer found the soft tissue to 68 million years ago. Many insisted the material she had found must be microbial contamination because no known process could account for such long preservation of organic material in bone, the molecules of which tend to be readily broken down and particularly for the preservation of its pliability and elastic qualities.
In ongoing studies, Schweitzer has discovered soft tissue and confirmed the presence of collagen in other dinosaur specimens alleged to be 145.5 to 199.6 million years old. Collagen is a structural protein commonly found in the connective tissue of many kinds of animals. In addition to demonstrating that there really are preserved protein molecules in some dinosaur bones, Schweitzer reported what may well be fragments of DNA.
Of course hemoglobin-containing blood circulates through the bodies of vertebrates, so there is an excellent opportunity for at least some cells and tissues to be exposed to iron soon after death. “We know that iron is always present in large quantities when we find well-preserved fossils,” Schweitzer says, “and we have found original vascular tissues within the bones of these animals, which would be a very hemoglobin-rich environment after they died.”4
The common thread running through “many exceptionally preserved fossils,”5 Schweitzer notes, is the presence of iron, which is found in hemoglobin. Iron atoms, once released from their bonds to hemoglobin molecules, would be highly reactive and Schweitzer hypothesizes that such iron “contributes to preservation in deep time, perhaps by both free-radical-mediated fixation and anti-microbial activity.”6 In other words, iron might stabilize biomolecular structures, deactivate enzymes that ordinarily break down tissue soon after death, and possibly inhibit bacterial degradation.
Putting Iron to the Test
To test whether iron such as that carried in hemoglobin can act as a preservative, Schweitzer’s team tested it on ostrich blood vessels. They soaked one batch of blood vessels in a hemoglobin solution7 and the other in water. The control group decayed within three days. The vessels soaked in the hemoglobin solution, produced by lysing red blood cells, “remained intact for more than 2 years at room temperature with virtually no change.”8 This represented a 200-fold increase in stability in the presence of hemoglobin, Schweitzer reports, confirming hemoglobin’s tissue fixation properties and supporting the possibility that iron could thus, under the right conditions, protect biomaterials (tissues, cells, and molecules) from degradation over deep time.9
Catastrophic Burial Still Essential
Preservation of soft tissue of course requires more that the presence of iron in a dead animal. Rapid burial is needed, and the global Flood supplied the rapid burial beneath huge masses of mineral-laden water-borne sediment requisite for the large-scale fossilization we see in earth’s sedimentary rocks. Burial in porous sediment through which water can flow, such as the sandstone in which soft-tissue-containing dinosaur bones have been found, is a further help to preserving delicate soft tissue. The bones of the specimens in which Schweitzer has found soft tissue have been articulated rather than scattered, which is additional testimony to their rapid burial. Thus rapid burial plus the release of biologically bound iron may be the twin keys to keeping dinosaur soft tissue around long enough for paleontologists to find it.
Stretching Back Through Deep Time
But can iron chelation preserve soft tissue and even keep it soft for millions of years? While a 200-fold delay in the decay of ostrich blood vessels is certainly impressive, even that level of preservation can’t hold a candle to the 99,800,000-fold10 increase in chemical stability needed in the millions-of-years evolutionary scenario. Schweitzer quite reasonably makes a comparison to the fixation properties of formaldehyde. Many variables influence the degree and duration of the decay-delaying properties of formaldehyde. But specimens preserved in formaldehyde are not preserved perfectly or permanently. While burial conditions likely influence the efficacy of iron as a preservative in any given bone, there is certainly no reason to propose that iron could preserve the molecular structure of soft tissue for millions of years any more than formaldehyde could.
The preservation of dead dinosaur tissue in a pliable state for the few thousand years since the global Flood demands an explanation.
Regardless of what anyone thinks is likely, the fact is it is impossible to scientifically test and observe the answer to this question. No scientist has ever observed the effects of millions of years on anything. The millions-of-years age assigned to the strata containing dinosaur fossils is derived from a number of worldview-based unverifiable assumptions. Therefore, the fact that dinosaur soft tissue is preserved in some fossils does not mean that iron or anything else has preserved it for millions of years. Iron chelation may be the (or a) key to preservation, a conclusion supported by Schweitzer’s work, but nothing in the discovery demonstrates how long such preservation could be effective.
When examined in light of the record of earth’s history recorded in God’s Word, much of the fossil record is easily understood as a record of the order of rapid catastrophic burial of billions of organisms during the year-long global Flood of Noah’s day about 4,350 years ago. Biology has never demonstrated any observable evolution of one kind of organism into a new, more complex one. Thus the fossil record is not a record of the evolution of life but much of it is the record of the order of burial as the global Flood overwhelmed the habitats of the world and sorted creatures in the deposition process.
Unlocking More Discoveries
Paradoxically, the destructive-preservative activity of these unleashed iron atoms may also obscure the proteins that remain in soft tissue, making it tricky for paleontologists to find preserved proteins in ancient fossil specimens. “We also know that iron hinders just about every technique we have to detect proteins,” Schweitzer explains. “So iron looks like it may be both the mechanism for preservation and the reason why we’ve had problems finding and analyzing proteins that are preserved.”11
Schweitzer and colleagues, in addition to identifying iron as the essential preservative in ancient soft tissues, found that applying a technique to un-chelate the iron and remove it made the preserved proteins more detectable. This technique may make it much easier to detect residual protein molecules in any newly discovered dinosaur fossils.
The iron-removal technique won’t likely be of any use in finding preserved proteins in fossils already found and stored, as they are typically treated with glues or chemical preservatives which are detrimental to the soft tissue. Furthermore, exposure to air contributes to degradation of soft tissue. Schweitzer plans to return to the field this summer in hopes of finding some nice new specimens on which to try out the technique. “I'd like to find a honking big T. rex that's completely articulated that's still in the ground, or something similar,” she says. “Once we can get the chemistry behind some of these soft tissues, there’s all sorts of questions we can ask of ancient organisms.”
Common Designs From a Common Designer—Our Creator
Scientists have in recent years identified several bio-molecules in ancient organisms. These include collagen in a mosasaur said to have been extinct for 65 million years and keratin in the skin of a lizard said to have been buried about 50 million years ago. The molecular structure of these proteins, like the protein detected by Schweitzer in dinosaur bone, are consistent as far as can be determined with the structures of their modern equivalents.
Many living things, past and present, share a number of the same biomolecules. This is no surprise, since all were designed by the same Creator to live in the same world. But finding ancient biomolecules that essentially match modern ones is not a demonstration of molecules-to-man evolution but only of the fact that all share a Common Designer.
Schweitzer writes, “Ironically, haeme, a molecule thought to have contributed to the formation of life, may contribute to preservation after death.”12 Her statement reflects the molecules-to-man evolutionary belief that life evolved from chemicals in spite of the degrading effects of oxygen because hemoglobin-like molecules evolved to make use of iron’s properties while preventing its harmful effects. Yet nothing in biological research has ever shown how the genetic information that blueprints the production of haeme-molecules and the countless other essential proteins in living things could spontaneously come into existence through random processes.
Molecules-to-man evolution is pure speculation without any observations to demonstrate its occurrence. Schweitzer, like other evolutionists, believes that molecules-to-man evolution must have occurred, not on the basis of scientific observations but on the basis of a worldview that rejects the eyewitness historical account of our Creator, a historical account that is completely consistent with the actual observations of biology.
Thus it may be ironic that hemoglobin, which protects living things from the harmful effects of the iron atoms they need, might contribute to the preservation of soft tissue in dead animals, but there is no observational reason to support the notion that hemoglobin enabled life to evolve.
We are certainly excited about the prospect of finding more examples of intact proteins or even DNA molecules. Hopefully Schweitzer’s iron-extraction technique will open the door to find much more. But finding preserved biomolecules in a dinosaur fossil does not and cannot demonstrate evolutionary ancestry. Preserved biomolecules resembling modern ones demonstrate neither evolutionary ancestry nor millions of years of history. Instead they are examples of the way our Creator employed many common designs to create an incredibly diverse biological world.
“Just The Facts, Ma’am”13
While Schweitzer’s original finding of dinosaur soft tissue was perhaps less of a shock to young earth creationists than to believers in millions of years, even the preservation of dead dinosaur tissue in a pliable state for the few thousand years since the global Flood demands an explanation.
Mary Schweitzer should be applauded for her team’s excellent detective work zooming in on that explanation. They deduced from observing iron’s ubiquitous presence in dinosaur soft tissue that iron might be a preservative. They determined, based on the observable biochemical behavior of iron, possible mechanisms showing how iron’s chemical behavior in life and death may enable it to function as a preservative. And most significantly Schweitzer’s team developed experimental support for the efficacy of iron as a postmortem preservative.
Further Reading
- Preservation of Cellular Proteins in Dinosaur Fossils
- Soft Tissue in Fossils
- More Soft Tissue in “Old” Fossils
- Two: Those Not-So-Dry Bones
- DNA Decay Rate Evaluated
- Doesn’t the Order of Fossils in the Rock Record Favor Long Ages?
- Radiometric Dating: Making Sense of the Patterns
- Radiometric Dating: Problems with the Assumptions
- Radiometric Dating: Back to Basics
Footnotes
- Mary H. Schweitzer et al., “A Role for Iron and Oxygen Chemistry in Preserving Soft Tissues, Cells and Molecules from Deep Time,” Proceedings of the Royal Society B 281, no. 1775 (January 22, 2014): doi:10.1098/rspb.2013.2741. See all footnotes
- Schweitzer et al., “A Role for Iron and Oxygen Chemistry in Preserving Soft Tissues, Cells and Molecules from Deep Time.” See all footnotes
- Tracey Peake, “Iron Preserves, Hides Ancient Tissues in Fossilized Remains,” NC State News, November 26, 2013, http://news.ncsu.edu/releases/schweitzer-iron. See all footnotes
- Peake, “Iron Preserves, Hides Ancient Tissues in Fossilized Remains.” See all footnotes
- Schweitzer et al., “A Role for Iron and Oxygen Chemistry in Preserving Soft Tissues, Cells and Molecules from Deep Time.” See all footnotes
- Ibid. See all footnotes
- The hemoglobin solution was prepared from chicken and ostrich blood. After lysing the red blood cells and concentrating purified hemoglobin, the hemoglobin was diluted to the concentration the researchers predicted it would have had in dinosaur blood, though it is impossible to know the actual concentration of hemoglobin in dinosaur blood. See all footnotes
- Schweitzer et al., “A Role for Iron and Oxygen Chemistry in Preserving Soft Tissues, Cells and Molecules from Deep Time.” See all footnotes
- Ibid. See all footnotes
- Schweitzer claims that she has found dinosaur soft tissue preserved in bones as old as 199.6 million years old. She has demonstrated that soaking in hemoglobin concentrate for 5 days can preserve blood vessels for 2 years. Do the math. If the observable preservation from a few days to 2 years constitutes a 200-fold increase in stability, then 199.6 million years would require a 99,800,000 increase in stability. In any case, neither Schweitzer nor any other scientist has the ability to test this idea, since the notion that dinosaurs lived millions of years ago instead of just thousands depends on worldview-based interpretation of the dating methods founded on unverifiable assumptions while disregarding the historical record contained in the Word of God. See all footnotes
- Peake, “Iron Preserves, Hides Ancient Tissues in Fossilized Remains.” See all footnotes
- Schweitzer et al., “A Role for Iron and Oxygen Chemistry in Preserving Soft Tissues, Cells and Molecules from Deep Time.” See all footnotes
- A line commonly attributed to classic television Detective Joe Friday in Dragnet, though it is actually a parody of his line, “All we want are the facts.” See all footnotes
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- © 2025 Answers in Genesis





