
Chapter 7
Doesn’t Carbon-14 Dating Disprove the Bible?
Scientists use a technique called radiometric dating to estimate the ages of rocks, fossils, and the earth. Many people have been led to believe that radiometric dating methods have proved the earth to be billions of years old. This has caused many in the church to reevaluate the biblical creation account, specifically the meaning of the word “day” in Genesis 1. With our focus on one particular form of radiometric dating—carbon dating—we will see that carbon dating strongly supports a young earth. Note that, contrary to a popular misconception, carbon dating is not used to date rocks at millions of years old.
Basics
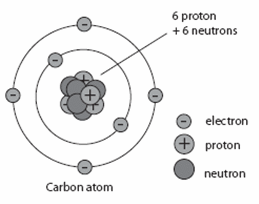
Before we get into the details of how radiometric dating methods are used, we need to review some preliminary concepts from chemistry. Recall that atoms are the basic building blocks of matter. Atoms are made up of much smaller particles called protons, neutrons, and electrons. Protons and neutrons make up the center (nucleus) of the atom, and electrons form shells around the nucleus.
The number of protons in the nucleus of an atom determines the element. For example, all carbon atoms have 6 protons, all atoms of nitrogen have 7 protons, and all oxygen atoms have 8 protons. The number of neutrons in the nucleus can vary in any given type of atom. So, a carbon atom might have six neutrons, or seven, or possibly eight—but it would always have six protons. An “isotope” is any of several different forms of an element, each having different numbers of neutrons. The illustration below shows the three isotopes of carbon.
Some isotopes of certain elements are unstable; they can spontaneously change into another kind of atom in a process called “radioactive decay.” Since this process presently happens at a known measured rate, scientists attempt to use it like a “clock” to tell how long ago a rock or fossil formed. There are two main applications for radiometric dating. One is for potentially dating fossils (once-living things) using carbon-14 dating, and the other is for dating rocks and the age of the earth using uranium, potassium and other radioactive atoms.
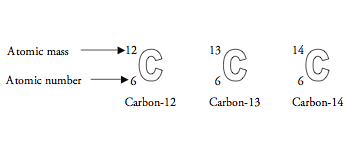
The atomic number corresponds to the number of protons in an atom. Atomic mass is a combination of the number of protons and neutrons in the nucleus. (The electrons are so much lighter that they do not contribute significantly to the mass of an atom.)
Carbon-14 Dating
Carbon-14 (14C), also referred to as radiocarbon, is claimed to be a reliable dating method for determining the age of fossils up to 50,000 to 60,000 years. If this claim is true, the biblical account of a young earth (about 6,000 years) is in question, since 14C dates of tens of thousands of years are common.1
When a scientist’s interpretation of data does not match the clear meaning of the text in the Bible, we should never reinterpret the Bible. God knows just what He meant to say, and His understanding of science is infallible, whereas ours is fallible. So we should never think it necessary to modify His Word. Genesis 1 defines the days of creation to be literal days (a number with the word “day” always means a normal day in the Old Testament, and the phrase “evening and morning” further defines the days as literal days). Since the Bible is the inspired Word of God, we should examine the validity of the standard interpretation of 14C dating by asking several questions:
- Is the explanation of the data derived from empirical, observational science, or an interpretation of past events (historical science)?
- Are there any assumptions involved in the dating method?
- Are the dates provided by 14C dating consistent with what we observe?
- Do all scientists accept the 14C dating method as reliable and accurate?
All radiometric dating methods use scientific procedures in the present to interpret what has happened in the past. The procedures used are not necessarily in question. The interpretation of past events is in question. The secular (evolutionary) worldview interprets the universe and world to be billions of years old. The Bible teaches a young universe and earth. Which worldview does science support? Can carbon-14 dating help solve the mystery of which worldview is more accurate?
The use of carbon-14 dating is often misunderstood. Carbon-14 is mostly used to date once-living things (organic material). It cannot be used directly to date rocks; however, it can potentially be used to put time constraints on some inorganic material such as diamonds (diamonds could contain carbon-14). Because of the rapid rate of decay of 14C, it can only give dates in the thousands-of-year range and not millions.
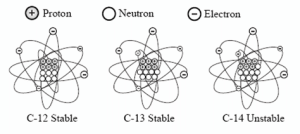
There are three different naturally occurring varieties (isotopes) of carbon: 12C, 13C, and 14C.
Carbon-14 is used for dating because it is unstable (radioactive), whereas 12C and 13C are stable. Radioactive means that 14C will decay (emit radiation) over time and become a different element. During this process (called “beta decay”) a neutron in the 14C atom will be converted into a proton. By losing one neutron and gaining one proton, 14C is changed into nitrogen-14 (14N = 7 protons and 7 neutrons).
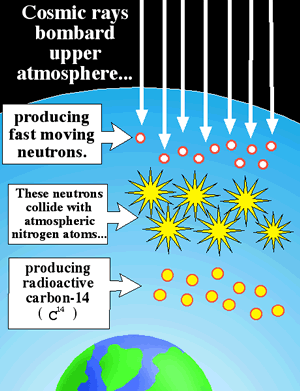
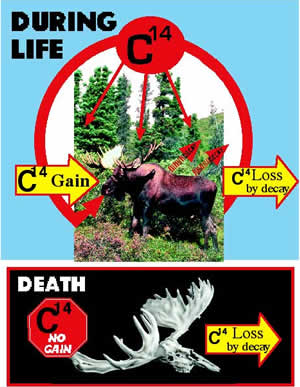
If 14C is constantly decaying, will the earth eventually run out of 14C? The answer is no. Carbon-14 is constantly being added to the atmosphere. Cosmic rays from outer space, which contain high levels of energy, bombard the earth’s upper atmosphere. These cosmic rays collide with atoms in the atmosphere and can cause them to come apart. Neutrons that come from these fragmented atoms collide with 14N atoms (the atmosphere is made mostly of nitrogen and oxygen) and convert them into 14C atoms (the neutron is accepted and a proton is ejected from the nucleus).
Once 14C is produced, it combines with oxygen in the atmosphere (12C behaves like 14C and also combines with oxygen) to form carbon dioxide (CO2). Because CO2 gets incorporated into plants (which means the food we eat contains 14C and 12C), all living things should have the same ratio of 14C and 12C in them as in the air we breathe.
How the Carbon-14 Dating Process Works
Once a living thing dies, the dating process begins. As long as an organism is alive it will continue to take in 14C; however, when it dies, it will stop. Since 14C is radioactive (decays into 14N), the amount of 14C in a dead organism gets less and less over time. Therefore, part of the dating process involves measuring the amount of 14C that remains after some has been lost (decayed). Scientists now use a device called an “Accelerator Mass Spectrometer” (AMS) to determine the ratio of 14C to 12C, which increases the assumed accuracy to about 80,000 years. In order to actually do the dating, other things need to be known. Two such things include the following questions:
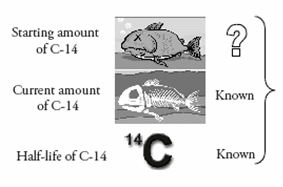
- How fast does 14C decay?
- What was the starting amount of 14C in the creature when it died?
The decay rate of radioactive elements is described in terms of half-life. The half-life of an atom is the amount of time it takes for half of the atoms in a sample to decay. The half-life of 14C is 5,730 years. For example, a jar starting with all 14C atoms at time zero will contain half 14C atoms and half 14N atoms at the end of 5,730 years (one half-life). At the end of 11,460 years (two half-lives) the jar will contain one-quarter 14C atoms and three-quarter 14N atoms.
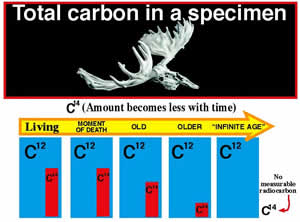
Since the half-life of 14C is known (how fast it decays), the only part left to determine is the starting amount of 14C in a fossil. If scientists know the original amount of 14C in a creature when it died, they can measure the current amount and then calculate how many half-lives have passed.
Since no one was there to measure the amount of 14C when a creature died, scientists need to find a method to determine how much 14C has decayed. To do this, scientists use the main isotope of carbon, called carbon-12 (12C). Because 12C is a stable isotope of carbon, it will remain constant; however, the amount of 14C will decrease after a creature dies. All living things take in carbon (14C and 12C) from eating and breathing. Therefore, the ratio of 14C to 12C in living creatures will be the same as in the atmosphere. This ratio turns out to be about one 14C atom for every 1 trillion 12C atoms. Scientists can use this ratio to help determine the starting amount of 14C.
When an organism dies, this ratio (1 to 1 trillion) will begin to change. The amount of 12C will remain constant, but the amount of 14C will become less and less. The smaller the ratio, the longer the organism has been dead. The following illustration demonstrates how the age is estimated using this ratio.
| Percent 14C Remaining | Percent 12C Remaining | Ratio | Number of Half-Lives | Years Dead(Age of Fossil) |
|---|---|---|---|---|
| 100 | 100 | 1 to 1T | 0 | 0 |
| 50 | 100 | 1 to 2T | 1 | 5,730 |
| 25 | 100 | 1 to 4T | 2 | 11,460 |
| 12.5 | 100 | 1 to 8T | 3 | 17,190 |
| 6.25 | 100 | 1 to 16T | 4 | 22,920 |
| 3.125 | 100 | 1 to 32T | 5 | 28,650 |
T = Trillion
A Critical Assumption
A critical assumption used in carbon-14 dating has to do with this ratio. It is assumed that the ratio of 14C to 12C in the atmosphere has always been the same as it is today (1 to 1 trillion). If this assumption is true, then the AMS 14C dating method is valid up to about 80,000 years. Beyond this number, the instruments scientists use would not be able to detect enough remaining 14C to be useful in age estimates. This is a critical assumption in the dating process. If this assumption is not true, then the method will give incorrect dates. What could cause this ratio to change? If the production rate of 14C in the atmosphere is not equal to the removal rate (mostly through decay), this ratio will change. In other words, the amount of 14C being produced in the atmosphere must equal the amount being removed to be in a steady state (also called “equilibrium”). If this is not true, the ratio of 14C to 12C is not a constant, which would make knowing the starting amount of 14C in a specimen difficult or impossible to accurately determine.
Dr. Willard Libby, the founder of the carbon-14 dating method, assumed this ratio to be constant. His reasoning was based on a belief in evolution, which assumes the earth must be billions of years old. Assumptions in the scientific community are extremely important. If the starting assumption is false, all the calculations based on that assumption might be correct but still give a wrong conclusion.
In Dr. Libby’s original work, he noted that the atmosphere did not appear to be in equilibrium. This was a troubling idea for Dr. Libby since he believed the world was billions of years old and enough time had passed to achieve equilibrium. Dr. Libby’s calculations showed that if the earth started with no 14C in the atmosphere, it would take up to 30,000 years to build up to a steady state (equilibrium).
If the cosmic radiation has remained at its present intensity for 20,000 or 30,000 years, and if the carbon reservoir has not changed appreciably in this time, then there exists at the present time a complete balance between the rate of disintegration of radiocarbon atoms and the rate of assimilation of new radiocarbon atoms for all material in the life-cycle.2
Dr. Libby chose to ignore this discrepancy (nonequilibrium state), and he attributed it to experimental error. However, the discrepancy has turned out to be very real. The ratio of 14C /12C is not constant.
The Specific Production Rate (SPR) of C-14 is known to be 18.8 atoms per gram of total carbon per minute. The Specific Decay Rate (SDR) is known to be only 16.1 disintegrations per gram per minute.3
What does this mean? If it takes about 30,000 years to reach equilibrium and 14C is still out of equilibrium, then maybe the earth is not very old.
Magnetic Field of the Earth
Other factors can affect the production rate of 14C in the atmosphere. The earth has a magnetic field around it which helps protect us from harmful radiation from outer space. This magnetic field is decaying (getting weaker). The stronger the field is around the earth, the fewer the number of cosmic rays that are able to reach the atmosphere. This would result in a smaller production of 14C in the atmosphere in earth’s past.
The cause for the long term variation of the C-14 level is not known. The variation is certainly partially the result of a change in the cosmic ray production rate of radiocarbon. The cosmic-ray flux, and hence the production rate of C-14, is a function not only of the solar activity but also of the magnetic dipole moment of the Earth.4
Though complex, this history of the earth’s magnetic field agrees with Barnes’ basic hypothesis, that the field has always freely decayed.... The field has always been losing energy despite its variations, so it cannot be more than 10,000 years old.5
Earth’s magnetic field is fading. Today it is about 10 percent weaker than it was when German mathematician Carl Friedrich Gauss started keeping tabs on it in 1845, scientists say.6
If the production rate of 14C in the atmosphere was less in the past, dates given using the carbon-14 method would incorrectly assume that more 14C had decayed out of a specimen than what has actually occurred. This would result in giving older dates than the true age.
Genesis Flood
What role might the Genesis Flood have played in the amount of carbon? The Flood would have buried large amounts of carbon from living organisms (plant and animal) to form today’s fossil fuels (coal, oil, etc.). The amount of fossil fuels indicates there must have been a vastly larger quantity of vegetation in existence prior to the Flood than exists today. This means that the biosphere just prior to the Flood might have had 500 times more carbon in living organisms than today. This would further dilute the amount of 14C and cause the 14C/12C ratio to be much smaller than today.
If that were the case, and this C-14 were distributed uniformly throughout the biosphere, and the total amount of biosphere C were, for example, 500 times that of today’s world, the resulting C-14/C-12 ratio would be 1/500 of today’s level. . . .7
When the Flood is taken into account along with the decay of the magnetic field, it is reasonable to believe that the assumption of equilibrium is a false assumption.
Because of this false assumption, any age estimates using 14C prior to the Flood will give much older dates than the true age. Pre-Flood material would be dated at perhaps ten times the true age.
The RATE Group Findings
In 1997 an eight-year research project was started to investigate the age of the earth. The group was called the RATE group (Radioisotopes and the Age of The Earth). The team of scientists included:
- Larry Vardiman, PhD Atmospheric Science
- Russell Humphreys, PhD Physics
- Eugene Chaffin, PhD Physics
- John Baumgardner, PhD Geophysics
- Donald DeYoung, PhD Physics
- Steven Austin, PhD Geology
- Andrew Snelling, PhD Geology
- Steven Boyd, PhD Hebraic and Cognate Studies
The objective was to gather data commonly ignored or censored by evolutionary standards of dating. The scientists reviewed the assumptions and procedures used in estimating the ages of rocks and fossils. The results of the carbon-14 dating demonstrated serious problems for long geologic ages. For example, a series of fossilized wood samples that conventionally have been dated according to their host strata to be from Tertiary to Permian (40-250 million years old) all yielded significant, detectable levels of carbon-14 that would conventionally equate to only 30,000-45,000 years “ages” for the original trees.8 Similarly, a survey of the conventional radiocarbon journals resulted in more than forty examples of supposedly ancient organic materials, including limestones, that contained carbon-14, as reported by leading laboratories.9
Samples were then taken from ten different coal layers that, according to evolutionists, represent different time periods in the geologic column (Cenozoic, Mesozoic, and Paleozoic). The RATE group obtained these ten coal samples from the U.S. Department of Energy Coal Sample Bank, from samples collected from major coalfields across the United States. The chosen coal samples, which dated millions to hundreds of millions of years old based on standard evolution time estimates, all contained measurable amounts of 14C. In all cases, careful precautions were taken to eliminate any possibility of contamination from other sources. Samples, in all three “time periods”, displayed significant amounts of 14C. This is a significant discovery. Since the half-life of 14C is relatively short (5,730 years), there should be no detectable 14C left after about 100,000 years. The average 14C estimated age for all the layers from these three time periods was approximately 50,000 years. However, using a more realistic pre-Flood 14C /12C ratio reduces that age to about 5,000 years.

These results indicate that the entire geologic column is less than 100,000 years old—and could be much younger. This confirms the Bible and challenges the evolutionary idea of long geologic ages.
Because the lifetime of C-14 is so brief, these AMS [Accelerator Mass Spectrometer] measurements pose an obvious challenge to the standard geological timescale that assigns millions to hundreds of millions of years to this part of the rock layer.10
Another noteworthy observation from the RATE group was the amount of 14C found in diamonds. Secular scientists have estimated the ages of diamonds to be millions to billions of years old using other radiometric dating methods. These methods are also based on questionable assumptions and are discussed elsewhere11. Because of their hardness, diamonds (the hardest known substance) are extremely resistant to contamination through chemical exchange. Since diamonds are considered to be so old by evolutionary standards, finding any 14C in them would be strong support for a recent creation.
The RATE group analyzed twelve diamond samples for possible carbon-14 content. Similar to the coal results, all twelve diamond samples contained detectable, but lower levels of 14C. These findings are powerful evidence that coal and diamonds cannot be the millions or billions of years old that evolutionists claim. Indeed, these RATE findings of detectable 14C in diamonds have been confirmed independently.12 Carbon-14 found in fossils at all layers of the geologic column, in coal and in diamonds, is evidence which confirms the biblical timescale of thousands of years and not billions.
Because of C-14’s short half-life, such a finding would argue that carbon and probably the entire physical earth as well must have a recent origin.13
Conclusion
All radiometric dating methods are based on assumptions about events that happened in the past. If the assumptions are accepted as true (as is typically done in the evolutionary dating processes), results can be biased toward a desired age. In the reported ages given in textbooks and other journals, these evolutionary assumptions have not been questioned, while results inconsistent with long ages have been censored. When the assumptions were evaluated and shown faulty, the results supported the biblical account of a global Flood and young earth. Christians should not be afraid of radiometric dating methods. Carbon-14 dating is really the friend of Christians, and it supports a young earth.
The RATE scientists are convinced that the popular idea attributed to geologist Charles Lyell from nearly two centuries ago, “The present is the key to the past,” is simply not valid for an earth history of millions or billions of years. An alternative interpretation of the carbon-14 data is that the earth experienced a global flood catastrophe which laid down most of the rock strata and fossils. . . . Whatever the source of the carbon-14, its presence in nearly every sample tested worldwide is a strong challenge to an ancient age. Carbon-14 data is now firmly on the side of the young-earth view of history.14
The New Answers Book 1
The New Answers Book 1 is packed with biblical answers to over 25 of the most important questions on creation,evolution, and the Bible.
Read Online Buy BookFootnotes
- Earth Science (Teachers Edition), Prentice Hall, 2002, 301.
- W. Libby, Radiocarbon Dating, Univ. of Chicago Press, Chicago, Illinois, 1952, 8.
- C. Sewell, “Carbon-14 and the Age of the Earth,” 1999. http://www.rae.org/pdf/bits23.pdf.
- M. Stuiver and H. Suess, On the relationship between radiocarbon dates and true sample ages, Radiocarbon, Vol. 8, 1966, 535.
- R. Humphreys, The mystery of earth’s magnetic field, ICR Impact, Feb 1, 1989. www.icr.org/article/292.
- J. Roach, National Geographic News, September 9, 2004.
- J. Baumgarder, C-14 evidence for a recent global Flood and a young earth, Radioisotopes and the Age of the Earth, Vol. 2, Institute for Creation Research, Santee, California, 2005, 618.
- A.A. Snelling, Radioactive “dating” in conflict! Fossil wood in ancient lava flow yields radiocarbon, Creation Ex Nihilo 20(1):24–27, 1997. A.A. Snelling, Stumping old-age dogma: Radiocarbon in an “ancient” fossil tree stump casts doubt on traditional rock/fossil dating, Creation Ex Nihilo 20(4):48–51, 1998. A.A. Snelling, Dating dilemma: Fossil wood in ancient sandstone: Creation Ex Nihilo 21(3):39–41, 1992. A.A. Snelling, Geological conflict: Young radiocarbon date for ancient fossil wood challenges fossil dating, Creation Ex Nihilo 22(2):44–47, 2000. A.A. Snelling, Conflicting “ages” of Tertiary basalt and contained fossilized wood, Crinum, central Queensland, Australia, Creation Ex Nihilo Technical Journal 14(2):99–122, 2000.
- P, Giem, Carbon-14 content of fossil carbon, Origins 51:6–30, 2001.
- Ibid., 587.
- Ibid., 609.
- M. Riddle, Does radiometric dating prove the earth is old?, in K.A. Ham (Ed.), The New Answers Book, Master Books, Green Forest, Arkansas, pp. 113–124, 2006.
- R.E. Taylor, and J. Southon, Use of natural diamonds to monitor 14C AMS instrument backgrounds, Nuclear Instruments and Methods in Physics Research B 259:282–287, 2007.
- D. DeYoung, Thousands ... Not Billions, Master Books, Green Forest, Arkansas, 2005, 61.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- Available Monday–Friday | 9 AM–5 PM ET
- © 2026 Answers in Genesis


